Breadcrumb
- Home
- Get Help
- Educational Tools
- DocuSign: Sending and Collecting Documents with eSignatures
DocuSign: Sending and Collecting Documents with eSignatures
Main navigation
- Alternatives to an In-Person Informed Consent Process for Non-FDA Regulated Studies
- Audio/Videotaping and Photographs of Research Subjects
- Consent Summary of Key Information
- Course-Related Student Research Projects Policy and Procedures
- Data Security Guidance
- Developing and Implementing Corrective and Preventative Action Plans
- DocuSign: Sending and Collecting Documents with eSignatures
- eConsent Checklist
- Exemption Tool
- FDA Site Inspection Guide
- HawkACT Checklist
- HSO/IRB Overview and Educational Resources
- Human Research Protection Program (HRPP) Committee Tool
- Human Research Protection Program Review Flow Chart
- Informed Consent Document Checklist (Biomedical Research)
- Informed Consent Document Checklist for Social, Behavioral, and Educational Research
- Investigational New Drug (IND) Guidance
- Investigational Device Exemption (IDE) Guidance
- PI Transfer/Departure Checklist
- Recruitment Letter Template
- Research Involving University of Iowa Student Records
- Secure Zoom Meetings and Recordings for Restricted and Critical Data
- Short Form Consent Document and Process
- Survey Based Research Educational Tool
- Umbrella Projects Policy and Procedures
This guidance document provides researchers with information about how to use DocuSign to collect valid electronic signatures (eSignatures) on Informed Consent Documents. DocuSign is a software program that captures electronic signatures and provides secure transfer and storage features. University of Iowa (UI) researchers across campus have access to DocuSign. The software is available for all desktop, laptop, and mobile devices. The University of Iowa DocuSign module cannot be used for FDA (Food and Drug Administration) regulated research.
Benefits of using DocuSign for eSignatures:
- Meets data security requirements for HIPAA (Health Insurance Portability and Accountability Act)
- Potential subjects do not need a DocuSign account
- Documents are sent via email and researchers can prohibit forwarding for confidentiality purposes
- Subjects receive a copy of the signed Informed Consent Document
UI researchers may activate a DocuSign account under the UI license.
The following sections guide researchers through the process of creating a document for eSignatures, sending and receiving the document, and maintaining an audit trail of the document.
Acronym Reminder:
- ICD = Informed Consent Document
- LAR = Legally Authorized Representative
- PWOC = Person Who Obtained Consent
Setting Up a DocuSign Account
The UI has a license for DocuSign. Contact ui-docusign-support@uiowa.edu to set up a DocuSign account. There are two versions of DocuSign. It is important to request the correct version for the type of data that will be collected:
1) for studies that contain protected health information (PHI) as defined under HIPAA (Health Insurance Portability and Accountability Act)
2) for studies that do not contain PHI as defined under HIPAA.
It is relatively quick and easy for researchers to set up a DocuSign account. Training is provided through the Purchasing Department. Once researchers set up a DocuSign account, they will need to activate it through the UI system. A UI DocuSign account is not connected to your HawkID.
Activate DocuSign
| Instructions | Screenshots |
Activate UI account
2. Log in or create a DocuSign account.
|


|
Create an Envelope
Currently, UI staff/departments are not charged to use DocuSign. The UI purchases “envelopes” for UI researchers to use. A DocuSign envelope is essentially an email, which will include the Informed Consent Document to capture electronic signatures. This video also gives a step-by-step guide.
Create an Envelope
| Instructions | Screenshots |
1. Click the Start button and the dropdown menu will appear 2. Choose “Send an Envelope” |
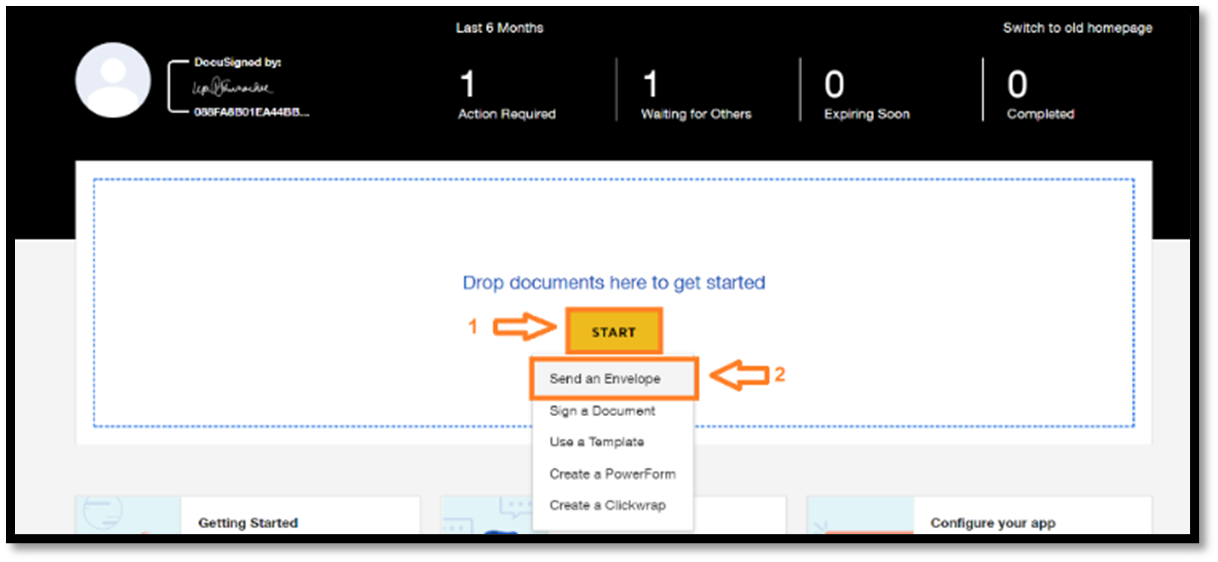
|
Add Documents, Recipients, and Template Email
For the DocuSign program to function properly, documents to be signed must be uploaded as PDFs. Converting an Informed Consent Document to a PDF does not affect the IRB approval stamp, therefore the DocuSign version of the consent document is compliant with UI policies and procedures. The DocuSIgn envenlope can also include additional reference materials that do not require a signature, such as a Consent Summary, diagrams, videos, etc.
- Add documents in the order that you want subjects to see them.
- Add anyone who needs to sign the Informed Consent Document as a recipient.
- If more than one person needs to sign the document, set the signing order.
Once the subject and/or the parent/legal guardian or Legally Authorized Representative (LAR) has signed the consent document, the person who obtains consent should receive the envelope for the final signature. The study team should ensure that processes are in place to identify the appropriate parent/guardian or LAR to route the Informed Consent Document for signature. The signature of the person who obtains consent should be the research team member who participated in the informed consent conversation and answered the subject’s questions about the study.
To ensure consistency for all DocuSign emails, the research team should use the same template language when sending the Informed Consent Document and for all reminders. The default is that the reminders use the same language, so researchers do not have to make any changes. DocuSign automatically sends three reminders. The research team can contact potential subjects once by phone if there is no response after the three reminders.
Email Template
Instructions: Copy and paste the following template language into the appropriate section in DocuSign. Replace the text in brackets with study-specific information.
[Salutation]
Thank you for thinking about enrolling in this study. [I am/We are] sending this Informed Consent Document to provide information and to describe what you will be asked to do if you decide to enroll in the study. You are not required to enroll in this study. [Insert the following statement if the study is clinical in nature.] Your clinical medical care will not be affected by whether you decide to enroll in this study.
If you do not sign this form, you will receive three reminders from DocuSign. We will attempt to call you if we do not receive a signed Informed Consent Document after the three reminders, to make sure you received the document and to see if you are interested in participating. If you let us know you are not interested in participating, we will not contact you again.
Please do not sign this form if you:
- Have more questions about this study that this Informed Consent Document does not answer or if you don’t understand something.
- If you would like to have a member of the study team go over this Informed Consent Document with you while you are reading it. (This may already be part of the consent process)
- Would like to talk with your family, friends, or medical provider before you agree to participate.
- Do not wish to participate in the study. There may be an option for you to decline in the document or you may contact the research team directly.
This video shows you how to use DocuSign, the program that allows you to sign/decline the Consent Document.
Please call or email [me / the research team] at [Insert phone number and email address]
[Insert signature block for the research team member or the study]
Add Documents, Recipients, and Template Email
| Instructions | Screenshots |
|
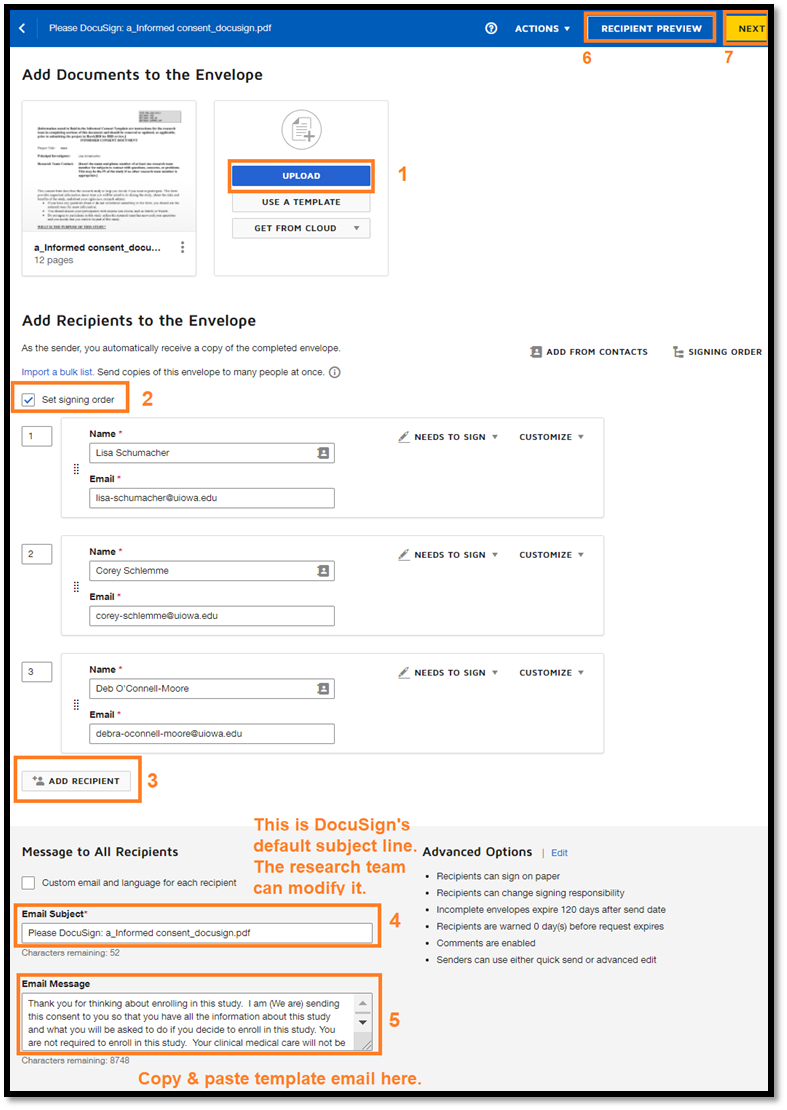
|
Setting Privileges, Reminders, and Expiration Date
| Instructions | Screenshots |
|

|
|
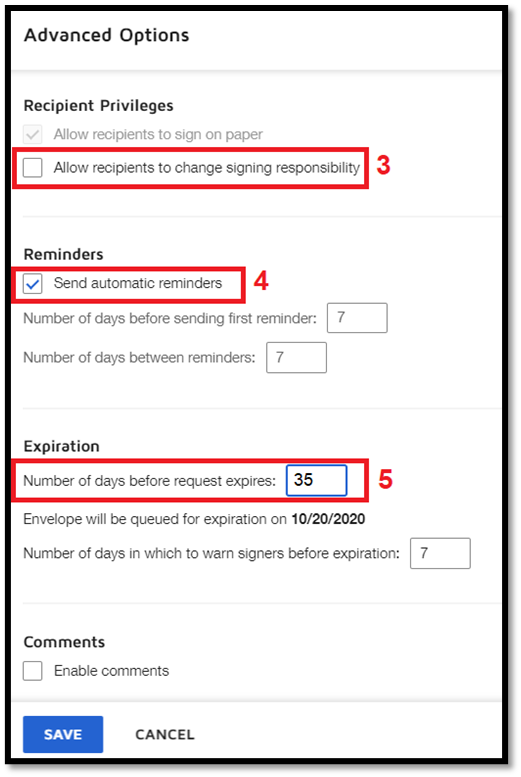
|
Designate Where to Initial, Sign, and Date the Informed Consent Document
DocuSign has the capability to allow subjects to select and initial optional agreements, sign and date the Informed Consent Document, and decline participation. These options are denoted by specific icons on the left side of the screen. The icons need to be placed exactly on the line where the subject must initial, sign or date. The research team may choose to add the “decline” icon next to the “signature” icon. Subjects should have the option to decline, even if they verbally agreed to participate during a conversation with a member of the research team. There are multiple ways to document that the subject declined. Indicating it in DocuSign provides an audit trail. If the subject clicks the decline icon, all reminders stop, and the research team will not follow up with a phone call.
Designate Where to Initial, Sign and Date the Informed Consent Document
| Instructions for Screenshot below |
|
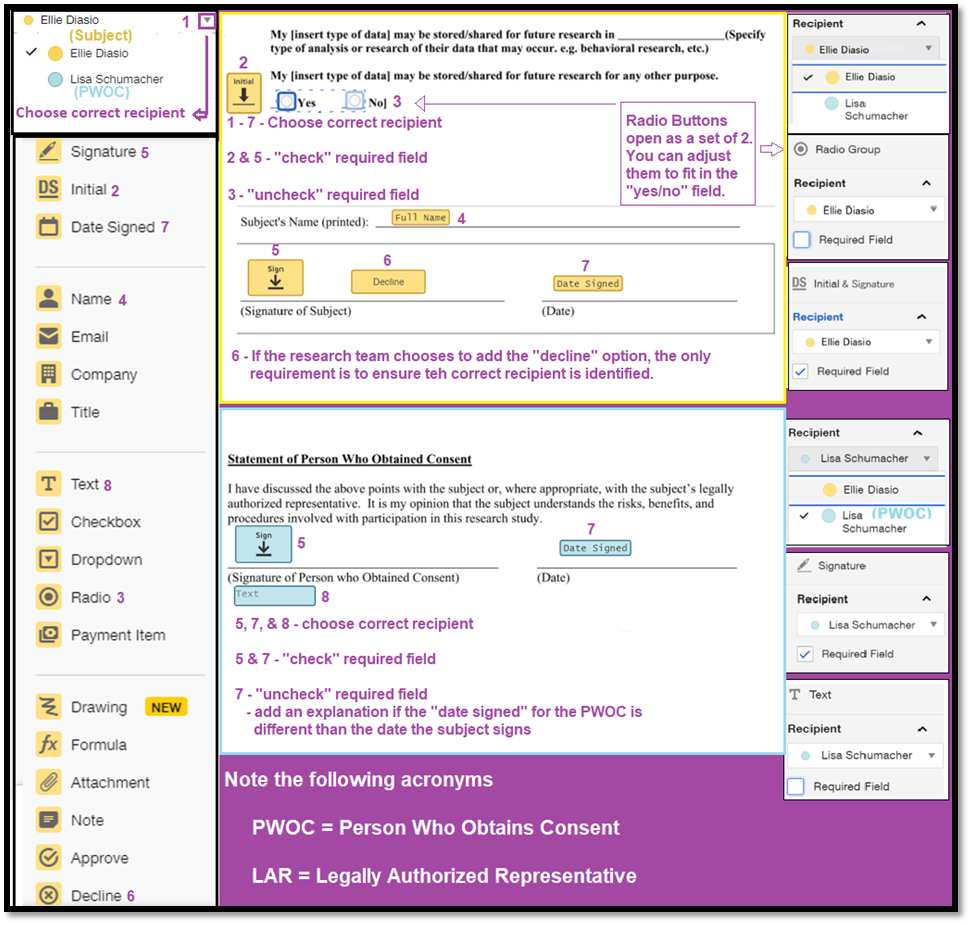
Send and Receive Documents
When the research team sends an Informed Consent Document for a subject to sign, the email contains the official DocuSign logo, a link to the document, and the customized email from the research team. Once the subject opens and reviews the document, DocuSign sends an automatic email to the research team. After the subject signs the Informed Consent Document, the research team and the subject receive an email that the process is complete with the signed document attached. There is a link to the DocuSign video in the template email to demonstrate the signing process.
What the Subject Sees
| Instructions | Screenshots |
|---|---|
| Subject line in subject’s email inbox |

|
This is the prompt subjects see when they open their email. Click on “Review Document” to go to next step. |
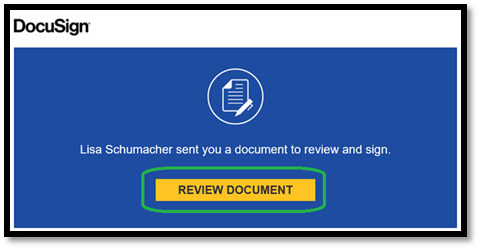
|
1. If the research team sets up an Access Code, subjects will get a prompt to enter the access code. 2. Click “validate” before they can view the Informed Consent Document. |

|
1.Subjects must agree to electronic signature disclosures. 2. Click “continue” to view Informed Consent Document. |

|
1. Subjects may decline. 2. Click “Finish” to complete the process after declining. |
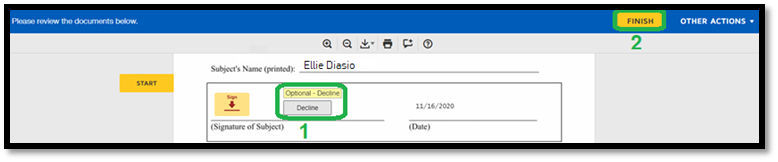
|
| This is the “safety” screen when subjects choose to decline. They must click “Continue” to complete the process. |
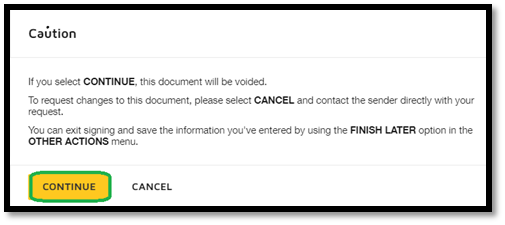
|
1. This checkbox does not need to be checked before subjects can complete the process to decline. 2. This completes the process of declining. |
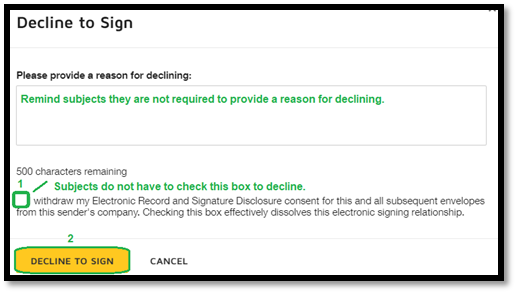
|
1. This is the first step in creating an electronic signature. 2. There are multiple signature styles subjects can choose, or they can draw their own signature. 3. Subjects must upload their signature. 4. Click “Adopt and Sign.” 5. Click “Finish” to complete the process. |
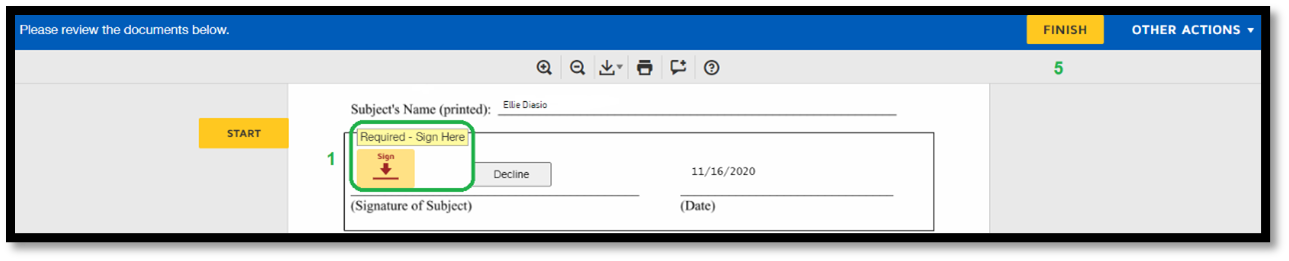

|
If subjects do not have a DocuSign account, this is the prompt they see once they sign. Subjects are not required to have a DocuSign account. 1. Subjects can download a copy of the signed Informed Consent Document. 2. Subjects can print the signed Informed Consent Document. 3. Click “Submit” to sign up for DocuSign account. 4. Click “No Thanks” to continue without a DocuSign account. |
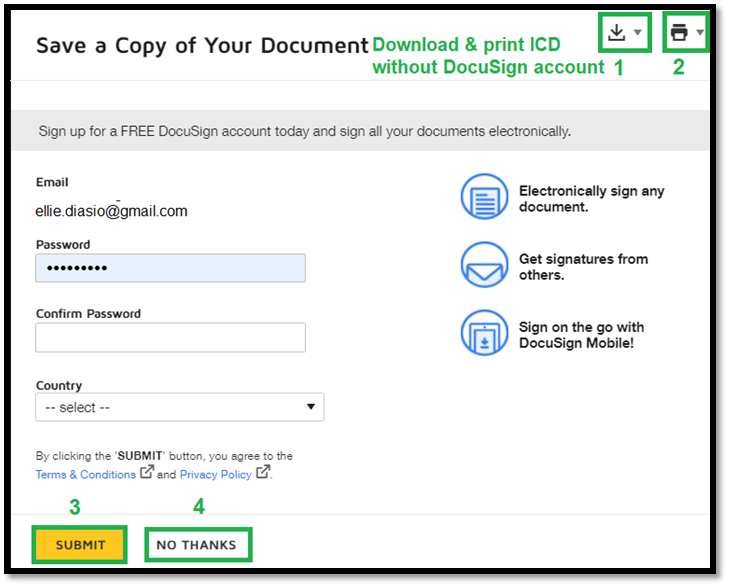
|
| This is the notice subjects see once they sign, and while they wait for the Person Who Obtained Consent to sign. |
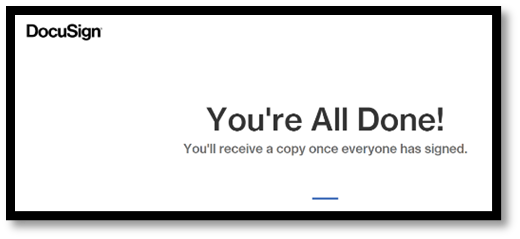
|
| Subject line in subject’s email inbox when all signatures have been captured. |

|
| Link to view completed document |
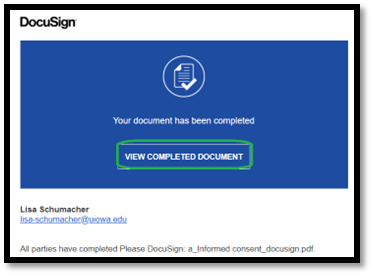
|
Data Security and Audit Trail
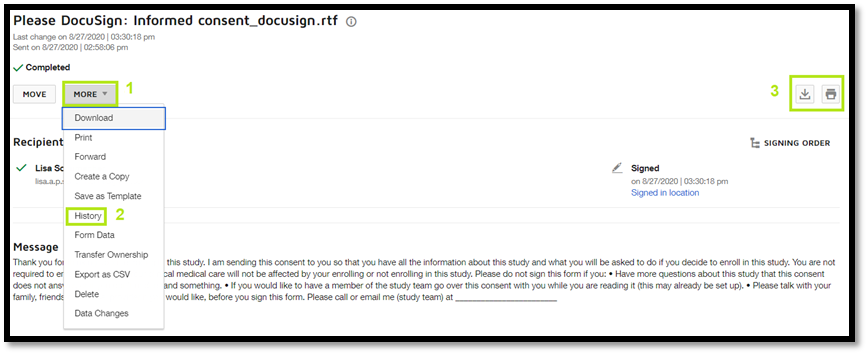
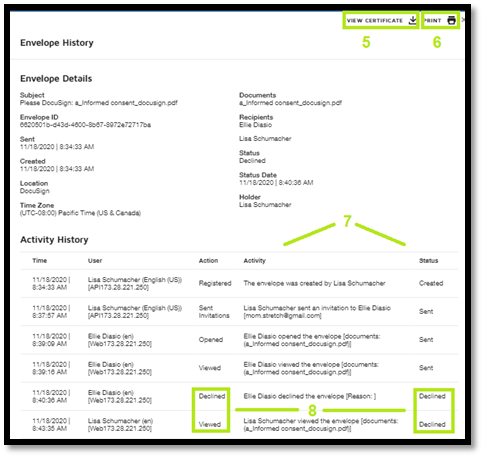
DocuSign provides secure storage of all documents sent and received through the program. The system also creates a Certificate of Completion with an audit trail of the Informed Consent Document, which includes the location of the persons who signed the document (i.e., subject, parent/guardian or LAR, and research team member who obtained consent).
Certificate of Completion
| Instructions | Screenshots |
|
|
|

|
Describe eConsent in the HawkIRB Application
| HawkIRB Section | eConsent Information |
|---|---|
| VII.D.8 | Answer “yes” if the consent process will be conducted through a video meeting over Zoom/Skype for Business. |
| VII.D.9 | Describe that the consent process is conducted through a video meeting over the internet, at a location of convenience for the subject. |
| VII.D.10 | Answer “yes” if the consent process will be conducted through an audio only call over the phone or by Zoom/Skype for Business. |
| VII.D.29 | Describe the eConsent process and documentation procedures for adult subjects, in detail.
|
| VII.D.30 | Describe the eConsent process and documentation procedures for minor subjects, in detail.
|
| X.4 | List the electronic systems used in the eConsent process and for documentation of consent. Describe the confidentiality protections/data security methods.
If Zoom will be used to meet with or record subjects, be sure to describe the IT security requirements as outlined in the Data Security Guidance and Secure Zoom Meetings and Recordings for Restricted Data. |
| Miscellaneous Attachments | Attach a document that contains the template email, which will be sent with the Informed Consent Document through DocuSign. |
| Informed Consent Document | Insert the following template language into the Informed Consent Document before the Person Who Obtained Consent signature line: Check the method by which consent is being obtained: ☐ Consent is being obtained electronically without a discussion between a research team member and the subject. (Research team member does not sign this document) ☐ Consent is being obtained electronically after a discussion between a research team member and the subject (in person or virtually). [End of template language] Instructions:
|
For printable version: DocuSign: Sending and Collecting Consent Documents with eSignatures for Human Subjects Research
Version 1, (3/25/22)