Breadcrumb
- Home
- Get Started
- ClinicalTrials.gov
ClinicalTrials.gov
Main navigation
ClinicalTrials.gov is a public database containing information about federally and privately supported clinical trials for an array of diseases and conditions. A service of the U.S. National Library of Medicine (NLM) at the National Institutes of Health (NIH), and in collaboration with the Food and Drug Administration (FDA), ClinicalTrials.gov requires registration and results reporting for Applicable Clinical Trials (ACT) and clinical trials wishing to share their findings with the public. Background information regarding history, policies, and laws can be found on ClinicalTrials.gov.
Section 801 of the Food and Drug Administration Act (FDAAA 801) specifies registration and results reporting requirements for clinical trials. These requirements were updated in 2017 under 42 CFR 11, also known as the Final Rule update to FDAAA 801. The Protocol Registration and Results System (PRS) is used to publish information on ClinicalTrials.gov and can be accessed at http://register.clinicaltrials.gov. This page outlines responsibilities requirements, and resolutions for ClinicalTrials.gov registration and results reporting at the University of Iowa (UI).
Contact Information
Protocol Registration and Results System (PRS) at University of Iowa
Fozia Ghafoor
ClinicalTrials.gov Compliance and Education Manager
Phone: 319-335-6564
Email: ct-gov@uiowa.edu
News and Updates
The U.S. Department of Health and Human Services has issued a final rule (42 CFR 11) about ClinicalTrials.gov. For more information, please see the HHS news release and NIH Office of Science Policy blog post. The Final Rule became effective on January 18, 2017 and required all institutions to comply no later than April 18, 2017. Institutions found in violation of this rule could be subject to civil penalties of up to $14,724, as well as an additional $14,724 per day, if the issue is not resolved within 30 days. Additionally, funding to the institution can be reduced or stopped, and non-compliance could impact future funding for the institution. Besides civil penalties, criminal penalties are also possible for investigators found in non-compliance of this rule. A checklist-based tool to assist responsible parties in evaluating whether a study is an Applicable Clinical Trial based on 42 CFR 11.22(b) is now available here.
ClinicalTrials.gov Resources
- How to Register a study on ClinicalTrials.gov
- Applicable Clinical Trial Checklist (ACT checklist)
- NIH & ICMJE Clinical Trial checklist
- HawkACT Checklist
New as of 08/26/2024: The ClinicalTrials.Gov Investigator's Guide has been updated. The ClinicalTrials.gov Investigator’s Guide covers the University of Iowa’s policy related to ClinicalTrials.gov registration and results reporting. This documentation satisfies the NIH requirement for data sharing, as well. A clean and tracked changes version are included to see recent changes. A summary of the recent updates is below:
- Section 8.0.3 Potential Consequences of Non-compliance has been updated; The Civil monetary penalties can be charged up to $14,724 per day.
Clinical Trial Reporting Requirements - Applicable Clinical Trial (ACT)
What is an ACT?
Studies required by law to register under federal regulations FDAAA 801 and 42 CFR 11 are known as Applicable Clinical Trials (ACT). To facilitate the determination of an ACT requiring registration, the NIH and ClinicalTrials.gov have adopted the ACT Checklist. All 4 points on this checklist must be ‘Yes’ for the study to be considered an ACT. The checklist provides ‘General Considerations’ which should be incorporated into an ACT determination.
Who determines if my study is an ACT
Given the breadth of these considerations, and the possible ramifications for misinterpreting the need to register, the UI has adopted a policy that requires the IRB to make the ACT determination. However, investigators can be involved in this determination by using the HawkACT Checklist, a simplified version of the official ACT Checklist, for UI use which can be submitted to the IRB.
What must I do if my study is an ACT
ACTs have a myriad of expectations to comply with the reporting requirements. For all studies determined to be an ACT, registration and results reporting is required. Additionally, study documentation must be uploaded to the ClinicalTrials.gov record when reporting results. The required documents include a written protocol, and a statistical analysis plan. As of January 21, 2019 this also includes a version of the informed consent document which was used to enroll subjects. There is also specific language required in the informed consent document to indicate the study is registered on ClinicalTrials.gov. This language can be found as part of the template informed consent document in HawkIRB.
How do I register an ACT on ClinicalTrials.gov
To register a study, investigators must use the Protocol Registration and Results System (PRS).To get started, investigators should contact a PRS administrator by emailing ct-gov@uiowa.edu to have an account created for them. Once a registered in the system, users will be able to add new projects in the PRS, or make updates to existing records, at any time. The University of Iowa stores and maintains their own records for ClinicalTrials.gov, but the review and publication of these records to ClinicalTrials.gov happens outside of the institution by the PRS team.
Other ClinicalTrials.gov Policies
Other policies regarding registration and results reporting have been adopted by some organizations in conjunction with the ClinicalTrials.gov Final Rule update, 42 CFR 11. These additional policies have been adopted to ensure studies funded by these organizations are represented on the site. Often, these separate policies are even more stringent than FDAAA 801 and 42 CFR 11. For journal publication specifically, it is recommended that each PI be aware of each of these rules, or to practice following the most stringent of these to assure compliance.
International Committee of Medical Journal Editors (ICMJE) – Publication Requirements
Manuscripts submitted to ICMJE journals for publication are required to register all clinical trials evaluating a drug, device, or behavioral intervention on ClinicalTrials.gov. View all journals supporting this requirement.
Additionally, Clinical trials that begin enrolling participants on or after 1 January 2019 must include a data sharing statement and subsequent plan in the trial's registration. ClinicalTrials.gov records satisfy this requirement through a registration module called Individual Participant Data (IPD) Sharing Statement. The module lets investigators identify ‘Yes’, ‘No’, of ‘Undecided’, but only a ‘Yes’ or ‘No’ will satisfy ICMJE’s requirement. Only records indicating ‘Yes’ will be required to share their data sharing plan. The ICMJE's policy regarding trial registration can be viewed on the ICMJE website. If the data sharing plan changes after registration this should be reflected in the statement submitted and published with the manuscript and updated in the registry record.
Studies wishing to publish in International Committee of Medical Journal Editors ICMJE journals may be required to register their clinical trial, even if not fully meeting other registration requirements. Investigators should be aware of the journals in which they would like to publish prior to starting a study and familiarize themselves with the journal’s requirements. Read the full ICMJE policy on the ICMJE website.
National Institutes of Health (NIH)
NIH requires all clinical trials, regardless of study phase, to register on ClinicalTrials.gov. The NIH definition of a ‘Clinical Trial’ applies to all studies which are interventional and involve prospective assignment of subjects to a group or groups to test the effect of the intervention on a biomedical, health-related, or behavioral outcome. This definition differs from the federal regulations in that a behavioral intervention, not just an evaluation of a drug or device, could be considered a clinical trial. More information on NIH policies related to clinical trials can be found on the NIH website.
World Health Organization (WHO)
All clinical trials of medical products utilizing funds from the World Health Organization (WHO) for research must register and report results of those clinical trials on ClinicalTrials.gov. Read more information WHO policy on their website.
Overview of ClinicalTrials.gov Reporting Policies
| Element | Final Rule | NIH Policy | ICMJE Policy |
|---|---|---|---|
| Scope/Applicability | Applicable clinical trials of FDA-regulated drug, biological, and device products and pediatric post-market surveillance studies of devices required by the FDA under the FD&C Act. Does not apply to phase 1 trials or small feasibility studies. Applicable clinical trials are (1) clinical trials of drug and biological products that are controlled, clinical investigations, other than phase 1 investigations of a product subject to FDA regulation; and (2) prospective clinical studies of health outcomes comparing an intervention with a device product against a control in humans (other than small feasibility studies) or any pediatric post-market surveillance studies required by FDA under the FD&C Act. Applies to public and private sector sponsors and other entities who meet the definition of a responsible party. | All clinical trials funded wholly or partially by NIH.
Includes phase 1 clinical trials and trials that do not involve any FDA regulated product such as trials involving only behavioral interventions.
Applies to NIH-funded clinical trials where applications or proposals are received by NIH on or after the policy’s effective date.
Applies to NIH-conducted clinical trials initiated on or after the policy’s effective date. | All clinical trials which wish to publish in an ICMJE journal, or its affiliates, must register prior to enrolling the first subject. As of July 1, 2018, manuscripts submitted to ICMJE journals must contain a data sharing statement. As of July 1, 2019, this statement must also be present in the ClinicalTrials.gov record. |
| Timeframe for registration on ClinicalTrials.gov | Not later than 21 days after enrollment of the first participant. | Not later than 21 days after enrollment of the first participant. | Prior to enrollment of first subject. |
| Registration data elements to be submitted to ClinicalTrials.gov | Elements defined in the final rule. Consists of descriptive information, recruitment information, location and contact information, and administrative data. | Elements defined in the final rule. Consists of descriptive information, recruitment information, location and contact information, and administrative data. | Elements defined in the final rule. Consists of descriptive information, recruitment information, location and contact information, and administrative data. |
| Timeframe for results information submissions to ClinicalTrials.gov | Not later than 12 months after primary completion date; possible delay of up to an additional 2 years for trials of unapproved products or of products for which initial FDA marketing approval or clearance is being sought, or approval or clearance of a new use is being sought. | Not later than 12 months after primary completion date; possible delay of up to an additional 2 years for trials of unapproved products or of products for which initial FDA marketing approval or clearance is being sought, or approval or clearance of a new use is being sought. | Not mandated in policy but must meet the requirements of FDAAA 801. |
| Results data elements to be submitted to ClinicalTrials.gov | Elements defined in the final rule. Includes participant flow, demographic and baseline characteristics, outcomes and statistical analyses, adverse events, the protocol, and statistical analysis plan, and administrative information. | Elements defined in the final rule. Includes participant flow, demographic and baseline characteristics, outcomes and statistical analyses, adverse events, the protocol, and statistical analysis plan, and administrative information. | Elements defined in the final rule. Includes participant flow, demographic and baseline characteristics, outcomes and statistical analyses, adverse events, the protocol, and statistical analysis plan, and administrative information. |
| Potential Consequences of Non-compliance |
|
|
|
Registration
Why should I register
Registration and results reporting is required by law for all Applicable Clinical Trials (ACT) based on the federal regulations (FDAAA 801 & 42 CFR 11). Severe penalties have been written into the registration requirement, which include:
- Fines exceeding $14,724 which can be charged per day if the issues is not resolved within 30 days
- Withholding or removal of funding
- Possible denial of future funding
- A notice of non-compliance in the public record
- Possible criminal prosecution
Some policies require registration separate from the reporting requirements of FDAAA 801 and 42 CFR 11. Some investigators choose to register to satisfy these policies which include the ability to publish in certain journals, or to satisfy funding requirements like NIH.
Who is required to register
Clinical trials registration and results reporting is required by law for all Applicable Clinical Trials, for clinical trials funded by NIH, and for investigators wishing to publish trial information in an ICMJE journal. The Responsible Party (sponsor) of a clinical trial is the person who initiates the trial. If this is a UI investigator, the term Sponsor-investigator identifies this role in the record. For trials sponsored by an industry sponsor, the industry sponsor is required to register and maintain the study record and must provide the NCT number to the UI Principal Investigator (PI). In the PRS, the term ‘Principal Investigator’ defines a situation where the industry sponsor designates a University of Iowa Principal Investigator as the responsible party or the record. If the sponsor is not a University of Iowa faculty or staff member, but is an investigator at another institution, the responsibility of reporting to ClinicalTrials.gov falls upon the sponsor’s institution to register and maintain the record..
If one of the following examples applies to you or your study, you are considered a sponsor-investigator:
- You hold the Investigational New Drug Application (IND) or the Investigational Device Exemption (IDE) for your study (responsible party = sponsor-investigator).
- Your study involves the UI serving as the coordinating center for a clinical trial under a prime award (as opposed to a subcontract) and the trial is investigator-initiated.
- You do not hold the IND or IDE for your study, but another UI investigator does and is not on your research team.
- The clinical trial is initiated by a UI investigator and regulated by the FDA (phase II through IV trial) but does not require an IND/IDE; and/or
- Your study involves a clinical trial and has NIH funding and was initiated by a UI investigator
Find more information at ClinicalTrials.gov under "Who is Responsible for Registering Trials and Submitting Results?"
When to register and update a record
The table below indicates when registration and results reporting on ClinicalTrials.gov apply to studies that meet the criteria of 42 CFR 11:
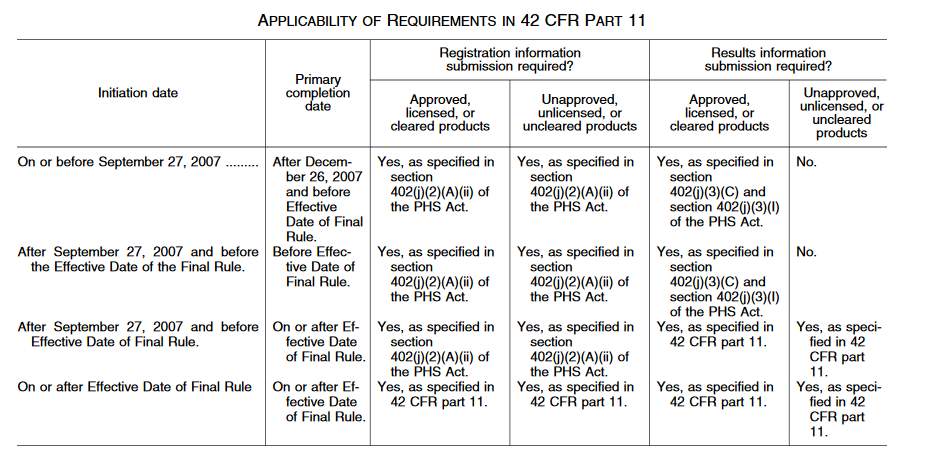
Applicable Clinical Trials (ACTs) and NIH funded trials are required to register on ClinicalTrials.gov within 21 days of enrollment of the first subject. Throughout the life of the record, updates must be made anytime the study plan changes, typically within 30 days. Updates are required at least every 12 months, even if nothing has changed. Additional information can be found on ClinicalTrials.gov “Which Trials Must Be Registered and Have Results Submitted to ClinicalTrials.gov?”. Trials planning to publish in ICMJE journals must register a study on ClinicalTrials.gov prior to enrolling the first subject. The table below outlines applicable deadlines
Data Element | Deadline for Updating (i.e., not later than the specified date) |
|---|---|
| Study Start Date | 30 calendar days after the first subject is enrolled |
| Overall Recruitment Status | 30 calendar days after a change in overall recruitment status.
|
| IRB Approval Status | 30 calendar days after a change in status. |
| Primary Completion Date | 30 calendar days after the clinical trial reaches its actual primary completion date. |
| Enrollment | At the time the primary completion date is changed to “actual,” the actual number of participants enrolled must be submitted. |
| Study Completion Date | 30 calendar days after the clinical trial reaches its actual study completion date. |
Responsible Party, by Official Title | 30 calendar days after a change in the Responsible Party or the official title of the Responsible Party. |
Responsible Party Contact Information | 30 calendar days after a change in the Responsible Party or the contact information for the Responsible Party. |
Device Product Not Approved or Cleared by U.S. FDA
| 15 calendar days after a change in approval or clearance status has occurred. |
| Record Verification Date | Any time the Responsible Party reviews the complete set of submitted clinical trial information for accuracy and not less than every 12 months, even if no other updated information is submitted at that time. |
What information do I need to register
Record registration is a summary of your study design and research plan. Below is a list of the data collected during the registration process, broken into separate modules in the PRS:
- Study Identification
- Study Status
- Sponsor/Collaborators
- Oversight
- Study Description
- Conditions
- Study Design
- Arms and Interventions
- Eligibility
- Contacts/Locations
- IPD Sharing Statement
- References
Where do I go to register
Registration on ClinicalTrials.gov happens in the Protocol Registration and Results System (PRS). Each institution has their own PRS account. To access the PRS, go to http://register.clinicaltrials.gov. The organization ID for the University of Iowa is ‘uiowa’.
Results Reporting
Results reporting on ClinicalTrials.gov is mandatory for all Applicable Clinical Trials (ACT) and for NIH-funded clinical trials. Results are reported as a summary of information collected, as well as the result of the self-identified ‘Outcome Measure’, or primary study aim, which was identified in the record registration.
When are results due
All Applicable Clinical Trials are required to report results within 1 year of the date that the final participant was examined or received an intervention for the purposes of final collection of data for the primary outcome, whether the clinical study concluded according to the pre-specified protocol or was terminated. This is required regardless of the funding source, presence of statistically significant findings, or journal publication status. Prior to submitting results data, it is recommended you review the “How to Submit Your Results” guidance on ClinicalTrials.gov.
What information do I need to report
Results are reported as a summary of the subjects enrolled, their movement through the study, and any adverse events which occurred. Finally, Outcome Measures which the Responsible Party identified in the registration of the record are summarized via self-generating charts. Any statistics used to analyze your results are also shared when reporting on the Outcome Measures. The following modules are used to enter the data in the results portion of the record:
- Participant Flow
- Baseline Characteristics
- Outcome Measures
- Adverse Events
- Limitations and Caveats
ClinicalTrials.gov Required Document Upload
At the time the sponsor-investigator is ready to submit results, the following documentation must be uploaded to the ClinicalTrials.gov record with a cover page identifying the Official Title, NCT number, and date the document was approved for use:
- Protocol
- Statistical Analysis Plan (SAP)
- Informed Consent Document
If the SAP is included with the Protocol, then an additional document is not needed. Documents must be uploaded in PDF/A format, which can be converted Adobe Acrobat Pro or similar program. If the PDF/A conversion cannot occur prior to upload, the PRS system is able to convert to this format for the investigator. However, any conversion done by the system should be reviewed for accuracy. As of January 2019, the Informed Consent Document is also required to be uploaded to the site for all federally funded clinical trials, a requirement of the Revised Common Rule. The informed consent document is only required for federally funded studies and should be uploaded after the clinical trial is closed to recruitment, and no later than 60 days after the last study visit by any subject, as required by the protocol.
Protocol Registration and Results System (PRS)
Introduction
The Protocol Registration and Results System (PRS) is the database where information published on ClinicalTrials.gov is entered. The PRS can be accessed by going to http://register.clinicaltrials.gov and logging into the UI account. The PRS consists of two sections: the Protocol Section (also known as the registration section) and the Results Section. Each section is broken down into modules where specific information is entered. The PRS offers help and advice for completing each module via the ‘Definitions’ and ‘Help’ links on each page. Additional information and training can be found on the ClinicalTrials.gov public site under the ‘Submit Studies’ tab. University of Iowa researchers should contact their PRS Administrator at ct-gov@uiowa.edu with additional questions. Please note the following University Policies when using the PRS:
- Users must input the IRB number (digits only) as the ‘Unique Protocol ID’
- Users should list themselves as ‘Sponsor-Investigator’
- The University of Iowa should not be listed as the sponsor for any study, unless otherwise instructed to do so
- The ‘Oversight’ module should list the IRB contact information as:
- Board Status: Approved Approval Number: HawkIRB ID No. (digits only)
- Board Name: University of Iowa IRB 01
- Board Affiliation: University of Iowa
- Phone: 319-335-6564 Email: irb@uiowa.edu
- Address:
Human Subjects Office / IRB
Hardin Library, Office 105
600 Newton Rd
Iowa City, IA 52242-1098
- Contact information should be for the study team and not the IRB
PRS Administrator
PRS administrator monitors records for the institution, provides help and feedback on using the system, creates and monitors accounts for the PRS, and informs investigators of issues needing resolution in the PRS system. Additionally, the PRS administrator reviews study applications involving clinical trials for the IRB and communicates relevant information to them.
Individuals applying for an account with ClinicalTrials.gov through UI, or who have questions should contact the PRS administrator:
- Protocol Registration and Results System (PRS) Administrator at UI
- Phone: 319-335-6564
- Email: ct-gov@uiowa.edu
PRS System information
Both Observational and Expanded Access Studies may be registered in ClinicalTrials.gov’s PRS; however, neither are considered Applicable Clinical Trials requiring registration. Please note that while expanded access studies are not Applicable Clinical Trials, if expanded access is offered for a drug or biologic, it must be noted in the record. Additionally, if an investigator both sponsors and manufactures an expanded access drug, they must create and maintain a record for that drug on ClinicalTrials.gov.
Institutional Policy
The Institutional Policy covers a wide-range of topics and concerns related to ClinicalTrials.gov. Some key components of the policy are discussed below.
PRS Record Listings
- Unique Protocol ID number – the ‘Unique Protocol ID’ field, the first field you enter in the record, should list the HawkIRB number. This connects the PRS record to the IRB record.
- Responsible Party (Sponsor-investigator) - There are three options for choosing the type of Responsible Party for a record: Sponsor, Principal Investigator, and Sponsor-Investigator. Each option identifies a different situation. In almost all cases, Sponsor-investigator should be selected to indicate the UI PI is both the sponsor who initiated the study protocol, and the investigator conducting the research.
IRB
The University of Iowa IRB considers the ClinicalTrials.gov requirements in its approval. To help assure that investigators are in compliance with federal regulations, the IRB will notify investigators when these regulations apply. The University and PRS Administrator recommend that investigators submit the HawkIRB application first to avoid needing to make additional changes to the PRS record. In addition, the IRB reviewers and PRS administrator will assist in assuring the record is updated appropriately. The NCT number in the IRB application must be entered in VII.B.12.b of the IRB application prior to IRB approval to show that the ClinicalTrials.gov record has been created. Likewise, the ClinicalTrials.gov record must list the IRB number as the ‘Unique Protocol ID’ so that the records can be linked.
HawkIRB
Section VII.B of the HawkIRB application is where you will identify your clinical trial and find the link to the ClinicalTrials.gov database to register your study. VII.B.1 should identify ‘Clinical Trial’ as the study type, and additional questions can be answered, as appropriate. There is a feature called “Push to PRS”. If PI has answered “Yes” to the question VII.B.12c in HawkIRB, then some of the information from HawkIRB approved application will be transferred to the PRS system and a new record will be created on PI’s behalf. This transfer will complete some sections of PRS application required for record registration. It is the responsibility of the PI to complete the remaining parts of the application.
VA Oversight of Investigator-initiated Clinical Trials
For studies where the VA is the primary study location or coordinating center of a study (listed in VII.A.10 of the IRB application), the study must be registered under the VA per their registration policy. Currently, the VA is requiring investigators who need to register at ClinicalTrials.gov under their institution to create and maintain their own institutional account in the Protocol Registration and Results System (PRS) for any records where the VA is the lead site. Accounts can be created by emailing a request to register@clinicaltrials.gov. Any study with both an IRB 01 and an IRB 03 application for the same study should look to VII.A.10 to identify which institution, the VA or UI, is the coordinating center of the study. Questions can be directed to Suzanne.kieffer@va.gov.
Need Help?
Find resources and tools on the Get Started page for ClinicalTrials.gov.