Breadcrumb
- Home
- Get Help
- Newsletters
- September 2022 IRB Connection Newsletter
September 2022 IRB Connection Newsletter
Student PI Training Requirement
Herky Hints: Need documentation of what changed in a Modification form? No problem! Here is how to generate a copy of a form and an approval memo
Announcement: Faster Access to Federally Funded Research Results
Reminder: Complete the Pre-Grant Submission Survey for Single IRB Model
Medical Ethics Advisor Newsletter, August 2022
In the News
Student PI Training Requirement
By Kelly O’Berry, BS, CIP
Graduate and undergraduate student Principal Investigators (PIs) are required to view recorded HawkIRB trainings (Parts 1 and 2) to learn how to navigate in the system and prepare thorough, detailed applications before they submit a New Project form in the HawkIRB system. Recorded trainings are posted in the IRB ICON Course for Researchers. Student PIs must view the recordings on regular speed and pass the quiz associated with each recording. This training requirement is intended to improve the quality of HawkIRB submissions and the efficiency of the IRB review process. This policy was implemented on August 23, 2021. It was originally announced in the June 2021 IRB Connection Newsletter, and via other UI campus communication channels.
HawkIRB System
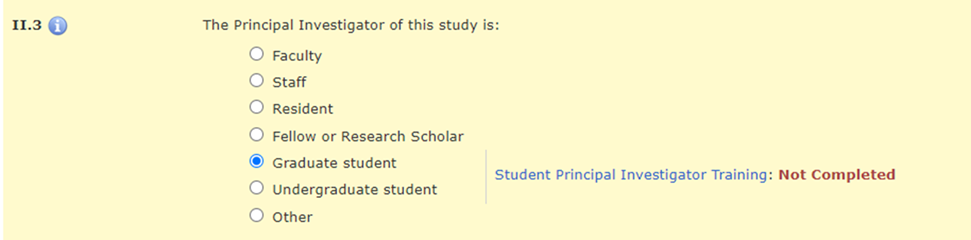
In Section II.3 of the HawkIRB application, when the PI selects “Graduate student,” “Undergraduate student,” or “Other,” there is a message with a hyperlink to the policy announcement and a statement about whether the required training was completed.
OR
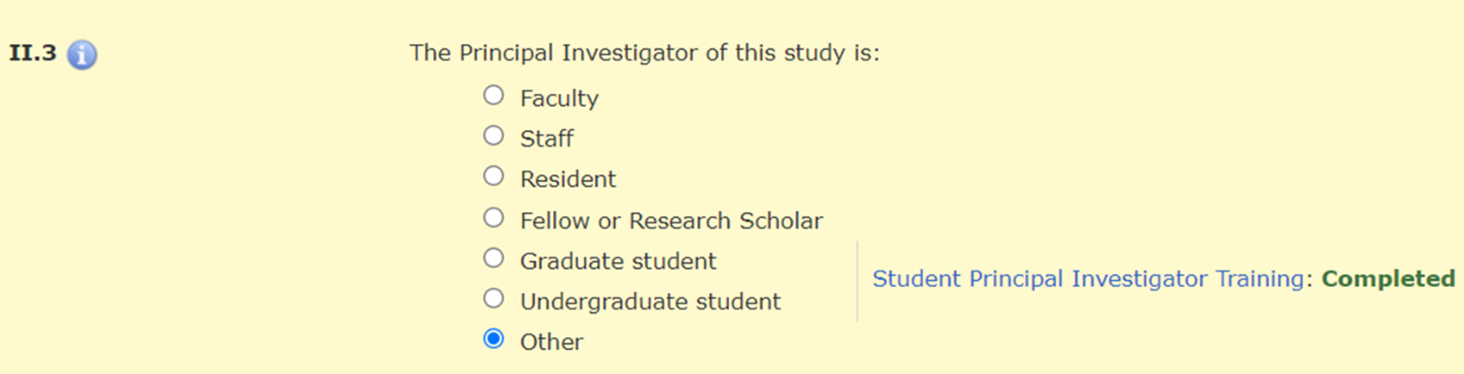
There is a hard stop that prevents a student PI from moving beyond Section II.3 until they complete this training requirement.

Although the training requirement is only for Parts 1 and 2, that cover the HawkIRB New Project form, student PIs are strongly encouraged to view these additional trainings:
- Part 3 - Forms Submitted After IRB Approval (Modification, Continuing Review, Reportable Event Forms, and Project Close Forms)
- Part 5 – Exempt Status HawkIRB Application (Many social/behavioral research projects and retrospective record reviews qualify for Exempt Status. See the Exemption Tool for more information about this type of research)
The student PI training is currently only required for students in the Graduate College and undergraduate students. However, we strongly encourage professional students, residents, Postdoctoral Fellows and Scholars and faculty and staff researchers and all HawkIRB Delegates to complete this training to learn how to prepare the best possible HawkIRB application. Thorough, detailed HawkIRB applications shortens the IRB review time for all researchers.
Training Options
Check the Education & Training page of the Human Subjects Office website for the portal to the IRB ICON Course for Researchers to access the recorded trainings. This ICON Course is available to anyone with a HawkID. The Human Subjects Office is not offering live/Zoom HawkIRB trainings for Fall 2022 semester, so recorded trainings are the only option.
Certified Investigator Database
Once a student PI views the recorded trainings and passes the quizzes, it takes up to two business days to be added to the Certified Investigator Database. Once added, the HawkIRB system will recognize that training is complete and allow the student PI to continue filling out the draft New Project form.
This training is required even if a student PI has already submitted a HawkIRB New Project form in the past. If a student PI previously attended the live HawkIRB trainings (in-person or via Zoom) but does not appear in the Certified Investigator Database, send the dates of attendance to irb-outreach@uiowa.edu. We will verify attendance at the HawkIRB New Project trainings (Parts 1 and 2) and update the database.
Questions or Comments
Please direct all questions/comments about the student PI training requirement to irb@uiowa.edu.
Herky Hints: Need documentation of what changed in a Modification form? No problem! Here is how to generate a copy of a form and an approval memo
By Rachel Kinker, MPA
You may need to print forms from HawkIRB to add to a regulatory binder, send to a sponsor or for your own record keeping. Fortunately, HawkIRB has a helpful function built in that allows research team members to pull a printer-friendly version of draft and approved forms. You can generate copies of Modification forms, Continuing Review forms and/or a general project summary. This function is valuable for sharing forms and maintaining a record of what was approved by the IRB, especially if someone will be leaving the UI.
Project Details
When you login to HawkIRB, you are automatically taken to the inbox with sections for workflow communication (Inbox), Draft Forms, Pending Forms and IRB-approved Projects. Click the IRB number for the project you would like to generate a printer friendly version of a form. On the Project Summary page under the History header at the bottom of the page, select the form name link. You will be directed to the Form Review tab. Click the link on the right, just below the tab header bar, to create a ‘printer friendly version of this form. This view opens in a new tab in your browser.

If you are a research team member, you will need to change the first filter in the Projects section of your HawkIRB inbox to view HawkIRB applications for which you are not the Principal Investigator.
You will continue to have access to all HawkIRB applications, even after they have been closed. Change the second filter in the Projects section to see projects that are ‘Closed.’
Approval Memos
To access the Approval memos, login to HawkIRB and click the IRB number of the approved project. Select the Approval tab and click the ‘Printer Friendly Version’ to print or save a pdf of the entire approval history. You can also print the individual Approval Memos on the Approval Tab.
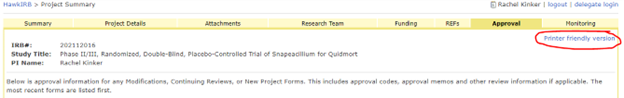
Modifications
You may need documentation of what specifically changed when you generated the modification. On the Project summary page, scroll down to the History section and click the link for the Modification form. This will direct you to the From Review tab. In the top right corner, select ‘View a printer friendly version of this form’.
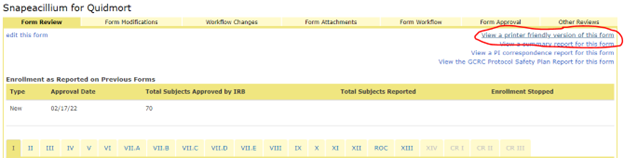
The view that is generated will show the original value and the new value for each question that was modified. After the Attachments section, this view shows the full application with those changes incorporated. This view opens in a new tab in your browser and can then be saved as an html file and emailed to the sponsor or printed and added to the regulatory binder.

Announcement: Faster Access to Federally Funded Research Results

In an August 25, 2022 memo to heads of executive departments and federal agencies, the White House Office of Science and Technology Policy (OSTP) announced policy guidance for them to update their public access policies by December 31, 2025. Data from federally funded research must be publicly available, without an embargo. The memo outlines the background and principles, justification and updates from the previous policy. Additional information is available in a White House press release and on the National Institutes of Health Director website.
Reminder: Complete the Pre-Grant Submission Survey for Single IRB Model

Federal agencies require the use of a single IRB (sIRB) for federally funded research conducted at multiple sites. This means one IRB oversees research conducted at some or all study sites. The budget must include the fees for this type of IRB review and there may be additional approvals and agreements for this type of research. Researchers can access information about UI IRB fees for budget planning.
For grant proposals to the National Institutes of Health (NIH) or any other federal agency that requires the sIRB model, complete the Pre-Grant Submission Survey as soon as you become aware of the award notice. Plan ahead and complete this survey well in advance, especially if the UI IRB will serve as the lead IRB or rely on an external IRB as an expectation of the grant. There are situations where the UI IRB cannot, or will not, serve as the lead/reviewing IRB (which will impact your study’s SIRB plan or budget).
If you have questions or need any assistance, contact the External IRB Team at uirb-external@uiowa.edu.
Medical Ethics Advisor Newsletter, August 2022
By Rachel Kinker, MPA

Medical Ethics Advisor (a publication of Relias, LLC) is a monthly newsletter with articles about human subjects research and medical ethics. Current and past issues of Medical Ethics Advisor and IRB Advisor are posted in the “IRB ICON Course for Researchers.” The portal to this ICON Course is on the Education and Training page of the Human Subjects Office website. This month we are spotlighting some articles about human subjects research from the August 2022 Medical Ethics Advisor Newsletter.
Wearable Tech in Clinical Research Trials
Researchers are partnering with companies to facilitate clinical research trials and developing their own wearable tech. The variability in the technology and how researchers utilize it for clinical trials raises unique considerations:
- Ethical concerns regarding collaboration with industry partners
- Informed consent, including repeated collection of personal health and behavioral data
- Privacy protection, consumer-based products are not regulated for clinical research, requiring considerations regarding third-party data sharing
- Risk assessment, unique nuances and risk associated with each technology
- Accessibility for the target population
- Researcher access to software used to collect and analyze large amounts of data
Parents, IRBs Hold Different Views on Phase I Pediatric Oncology Trials
In Phase I studies for pediatric cancers, researchers often enroll children that have not performed well on other treatments or when there are no other available standard treatments. These trials can carry additional risk and it may be unclear if there will be any benefit for the participants at the time of enrollment. For this reason, Phase I pediatric oncology trials are often ethically controversial. Parents/caregivers are often focusing on any potential treatment that could help the child and are more likely to think the potential benefits justify the risk.
IRBs are tasked with ensuring that trials are ethical and that pediatric subjects are not exposed to excessive risk, possibly leading IRB members to conclude that risks exceed benefits. Researchers must weigh these perspectives when designing study protocols, potentially utilizing parents/caregivers as consultants to provide a unique perspective.
New Guidance on Incorporating Patient-Reported Outcomes in Clinical Research
Patient-reported outcomes (PROs) are commonly utilized in clinical trials to track how a patient feels and functions. As Patient Reported Outcomes are incorporated into study protocols, they need ethical guidance.
Considerations for incorporating Patient Reported Outcomes;
- Does the trial include clear rationale for the Patient Reported Outcomes selected? When will it be administered and how will the data be used?
- Will the care team be notified about concerning symptoms, if so, when, and how?
- How will equitable access to Patient Reported Outcome symptoms be monitored and mitigated?
- Do consent forms address how Patient Reported Outcome data will be collected, how the patient will be contacted to completed Patient Reported Outcomes and other relevant information?
- Do all participants have access to participate in Patient Reported Outcomes?
- How will concerning levels of psychological stress or physical symptoms reported in Patient Reported Outcomes be addressed and addressed consistently?
Articles in the August 2022 Issue:
- Cardiac Xenotransplantation Could Fill the Organ Donor Gap, But Is It Ethical?
- Ethics Plays Important Role in Response to Abortion Ruling
- Clinicians Must Remain Cautious When Using social media
- Pain Researchers Are Engaging Patients as Partners
- Ethics Consults During Pandemic Inform Preparation for Future Crises
- Few People with Limited English Proficiency Participate in Stroke Studies
- Updated Guidance Provides Sense of Urgency to Improve Clinical Trial Diversity

In the News, September 2022

- It’s Time to Rethink the Origins of Pain, Scientific American
- A New Theory for Gene Ownership, Harvard Law Bill of Health
- Two surprising reasons behind the obesity epidemic: Too much salt, not enough water, The Conversation
- Team developing oral insulin tablet sees breakthrough results, ScienceDaily
- Meditation holds the potential to help treat children suffering from traumas, difficult diagnoses or other stressors-a behavioral neuroscientist explains, The Conversation
- Yes, Black patients do want to help with medical research-here are ways to overcome the barriers that keep clinical trials from recruiting diverse populations, The Conversation
- Learning to control microglia using CRISPR, NIH
- Clinical Trial Evaluating Monkeypox Vaccine Begins, NIH
- New study links ultra-processed foods and colorectal cancer in men, ScienceDaily