Breadcrumb
- Home
- Get Help
- Newsletters
- August 2023 IRB Connection Newsletter
August 2023 IRB Connection Newsletter
Course-Related Student Projects: The Exception to the Rule
Student PI Training Requirement: Reminder to Faculty Advisors and Student PIs
Best practices: Studies Involving LGBTQIA+ Persons
Informed Consent – New Food and Drug Administration (FDA) Guidance
Medical Ethics Advisor Newsletter, July 2023
In the News
Course-Related Student Projects: The Exception to the Rule
By Rachel Kinker, MPA
We want research methods course instructors to know about one important exception to the rule that research involving human participants requires IRB approval. This is especially relevant for research methods courses where students conduct research projects with human participants as a course assignment. As a flexibility initiative to reduce regulatory burden, UI IRB policy establishes criteria for certain projects conducted as a course requirement to be done without IRB approval.
Policy and Checklist
The Course-Related Student Project policy (UI IRB Standard Operating Procedures and Researcher Guide, Section I, Part 12.D) specifies the parameters for class projects that can be conducted without IRB approval. There is a related checklist to help students and their instructors know when IRB approval might be required. It depends on the intent or purpose of the project, the design and procedures, the subject population, and how data from the project will be used.
According to the UI Operations Manual and IRB policies, all human subjects research conducted by University of Iowa faculty, staff or students must have approval from the Institutional Review Board (IRB) prior to initiation. This is required if the project is intended to “develop or contribute to generalizable knowledge,” including thesis or dissertation projects. However, research methods course projects are generally more limited in scope and are intended to help students learn how to conduct research. These projects satisfy curriculum requirements and are not intended to further scientific knowledge in a particular field or discipline.
Honor’s, and master’s thesis or doctoral dissertation projects that meet the regulatory definition of human subjects research will always require IRB review and approval. If a course-related project will be used to support a thesis or dissertation project, then the student researcher should obtain IRB approval prior to conducting the project.
IRB Approval Not Required
Course-related research activities would not require IRB approval if:
- The purpose of the assignment is to teach research methodology.
- The results of the assignment will not “contribute to generalizable knowledge” because they are either being used to satisfy a course requirement, or because of limits on who will have access to the results of the project.
- The procedures will be limited to surveys, questionnaires, interview procedures, observation of public behavior, or standard educational exercises.
- The participants will not include prisoners, children, or data about them.
- Data will be recorded without any identifying information (such as code numbers, birth dates, etc.). Or the data are not sensitive in nature (i.e., do not pose a risk of harm to the participants’ reputation, employment, financial standing, or do not put them at risk for criminal or civil liability).
- There is no monetary compensation or direct financial support for the project from an external company, organization, or agency.
- The project will not be conducted at the Veteran’s Administration Health Care System (VAHCS) or using any VA resources.
- The project is not conducted or supported by a federal department or agency that follows the federal regulations for the protection of human subjects (the ‘Common Rule’).
The Course-Related Student Projects Checklist is a fillable pdf that students complete and instructors use to determine whether a project qualifies as a course-related student project. If any aspect of the project design indicates that IRB approval might be required, a pop-up message directs the student to submit a Human Subjects Research Determination form in the HawkIRB system to ask if the project needs IRB approval.
The student should submit the completed checklist to the course instructor. The instructor should review it for accuracy and confirm whether the project qualifies for the exemption or if the student should submit an HSRD form.
Information for Participants
Even when a course-related student project can be conducted without IRB approval, the student should share the following information with potential subjects:
- Student name and name of the course
- Course instructor name and their contact information
- Who will have access to individual and summarized results (e.g. instructor, group/team members, the whole class, an outside company/agency/organization)
- That participation is voluntary, and they may stop participating at any time.
Guidance and IRB Overview Presentation
For additional guidance, see the Course-Related Student Project Educational Tool. We can provide an IRB overview presentation about human subjects research:
- What does and does not need IRB approval
- Research ethics
- Criteria for IRB approval
- How to ask if you need approval (HSRD form)
- IRB review process
- Other topics upon request
There is now a recording of the IRB Overview presentation available in the ICON Course for Researchers. This can be used as a homework assignment or shown during a class period. If an in-person presentation is preferred, contact the IRB Education & Outreach Program(link sends e-mail).
Student PI Training Requirement: Reminder to Faculty Advisors and Student PIs
Graduate and Undergraduate student Principal Investigators (PIs) are required to view recorded HawkIRB trainings (Part 1 and 2) to learn how to navigate in the HawkIRB system and prepare thorough, detailed HawkIRB applications. Faculty Advisors and others who work with student Principal Investigators: Please make sure graduate and undergraduate student PIs know to complete this training in the IRB ICON Course for Researchers.
This training is required for students in the graduate college and undergraduate students who will serve as the PI of their own project. This training is strongly encouraged for faculty and staff, postdocs, residents, trainees (Medicine, Dentistry, Pharmacy) and HawkIRB Delegates to learn how to prepare the best possible HawkIRB application. Thorough, detailed HawkIRB applications shortens the IRB review time for all researchers.
Student PIs must view the recordings on regular speed and pass the quiz associated with each recording. This training requirement is intended to improve the quality of HawkIRB submissions and the efficiency of the IRB review process. This policy was implemented on August 23, 2021. It was originally announced in the June 2021 IRB Connection Newsletter, and via other UI campus communication channels.
HawkIRB System
In Section II.3 of the HawkIRB application, when the PI selects “Graduate student,” “Undergraduate student,” or “Other,” there is a message with a hyperlink to the policy announcement and a statement about whether the required training was completed.
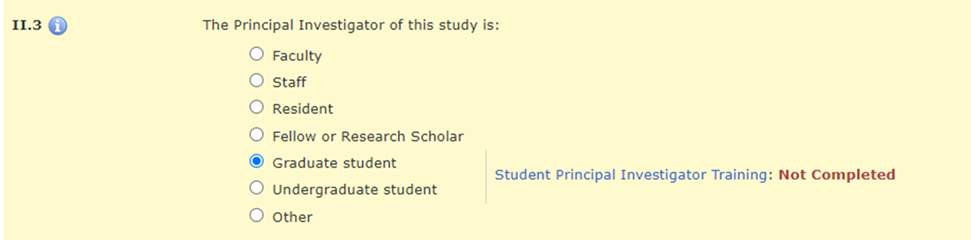
OR
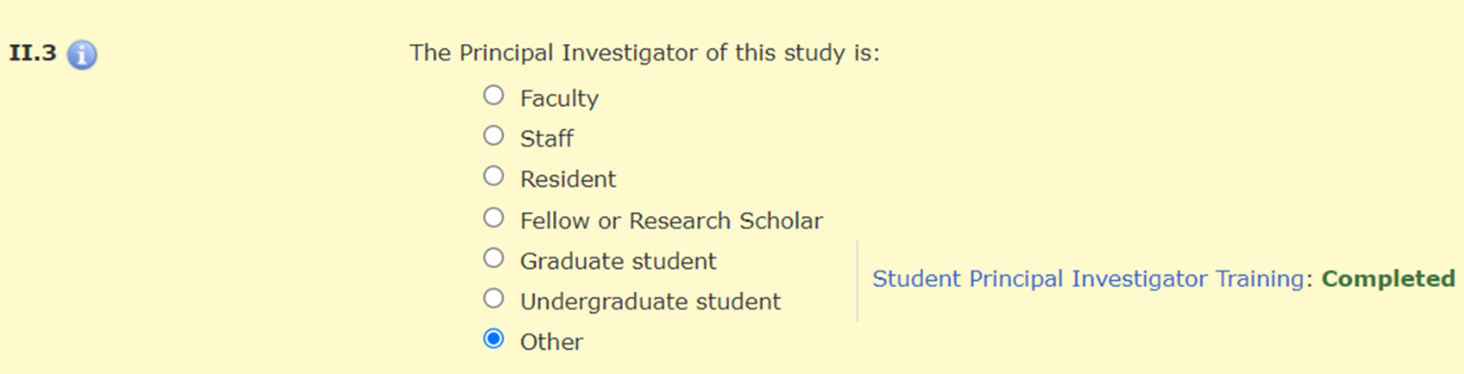
There is a hard stop that prevents a student PI from moving beyond Section II.3 until they complete this training requirement.

Although the training requirement is only for Parts 1 and 2, that cover the HawkIRB New Project form, student PIs are strongly encouraged to view these additional trainings:
- Part 3 - Forms Submitted After IRB Approval (Modification, Continuing Review, Reportable Event Forms, and Project Close Forms)
- Part 5 – Exempt Status HawkIRB Application (Many social/behavioral research projects and retrospective record reviews qualify for Exempt Status. See the Exemption Tool for more information about this type of research)
Training Options
Check the Education & Training page of the Human Subjects Office website for the portal to the IRB ICON Course for Researchers to access the recorded trainings. This ICON Course is available to anyone with a HawkID. The Human Subjects Office is not currently offering live/Zoom HawkIRB trainings, so recorded trainings are the only option.
Certified Investigator Database
Once a student PI views the recorded trainings and passes the quizzes, it takes up to three business days to be added to the Certified Investigator Database. Once added, the HawkIRB system will recognize that training is complete and allow the student PI to continue filling out the draft New Project form.
Questions or Comments
Please direct all questions/comments about the student PI training requirement to irb@uiowa.edu.(link sends e-mail)
Best practices: Studies Involving LGBTQIA+ Persons
By Shane D. Soboroff, PhD
Researchers have a responsibility to consider the needs and dignity all persons engaged in research. In 2015, The National Institutes of Health launched The Sexual & Gender Minority Research Office (SGMRO), which coordinates sexual and gender minority (SGM)–related research
and activities by working directly with the NIH Institutes, Centers, and Offices. One of the office’s goals is to advance rigorous research on the health of SGM populations in both the extramural and intramural research communities. Historically, SGM persons have been harmed by medical and/or socio-behavioral research that treats them as abnormal or problematic. This article discusses best practices for working with SGM communities, designing inclusive studies, and preparing IRB applications for research that could include SGM populations.
Sex, Gender, and Sexuality as Categories and Identities
According to the NIH, SGM populations include, but are not limited to, “individuals who identify as lesbian, gay, bisexual, asexual, transgender, Two-Spirit, queer, and/or intersex. Individuals with same-sex or -gender attractions or behaviors and those with a difference in sex development are also included. These populations also encompass those who do not self-identify with one of these terms but whose sexual orientation, gender identity or expression, or reproductive development is characterized by non-binary constructs of sexual orientation, gender, and/or sex.”
Modern science studies a variety of distinctions related to sex, sex category, gender, and sexuality that are known to impact human behavior and health. Research to understand variability on these characteristics has led to improvements in medical, psychological, social, and educational practices. We can (imperfectly) define these characteristics as follows:
- Biological sex: Genetic expression of chromosomes affecting physical characteristics and reproductive ability, among other traits.
- Sex category: categorization of a human being as male, female, or intersex based on physical sex characteristics, usually assigned at birth.
- Gender: Widely shared expectations and traits associated with culturally recognized sex categories.
- Gender identity: An individual’s cognitive and emotional self-definition with respect to gender and sex categories.
- Sexuality: An individual’s orientation towards sexual and/or romantic relationships, including desired types of relationships and desirable traits in partners.
Ethical Research Design that is Mindful of SGM Populations
Specific disciplines may have historical conventions for gathering data on SGM populations. There may also exist sound rationale for focusing on a particular subset of categories related to sex, sex category, gender, gender identity, or sexuality, especially in biomedical research.
Researchers should consider the following questions when designing research that includes members of SGM populations:
- Am I studying physical sex characteristics or socially constructed gender categories? Will subjects be able to provide the information I’m seeking?
- Will the study enroll subjects from SGM populations, and is my rationale for their inclusion or exclusion scientifically sound?
- If enrolling subjects from SGM populations and/or gathering data on sex/gender/sexuality, are my response categories exhaustive and/or have I allowed the subjects to self-identity in an appropriate way?
- Should I consult with members of the population under study given the research question and sensitivity of information sought?
- What harms might result from divulging private, identifiable information about members of SGM populations, and what steps can I take to mitigate risks?
UI IRB Review of Research with SGM Populations
IRB approval criteria reflect the broad principles of Respect for Persons, Beneficence, and Justice outlined in the Belmont Report. Human subjects research has historically included cases of injustice against people from SGM populations. While SGM populations are not specifically identified as ‘vulnerable’ under federal guidelines, there are potential risks to subjects from SGM populations should their personal information be disclosed.
The HawkIRB application should address the following questions to facilitate the IRB review process:
- Are questions regarding sex, sex category, gender, gender identity, and/or sexuality written in a way that allows accurate representation of the populations being enrolled?
- Do informed consent documents clearly state risks from disclosure of sensitive information, as well as methods for mitigating these risks?
- If there is overlap between SGM populations and vulnerable populations identified in federal regulations (e.g. LGBTQ+ children), how will consent/assent procedures be conducted in a way that protects subject privacy and confidentiality?
- Do inclusion criteria and screening procedures burden SGM populations with risks or exclude SGM populations from benefits without strong scientific rationale?
Additional Guidance
Guidance from Northwestern University and WCG expands on the information provided here and offers suggestions for researchers and IRBs considering research with SGM populations.
Informed Consent – New Food and Drug Administration (FDA) Guidance
On August 15, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance, "Informed Consent: Guidance for IRBs, Clinical Investigators, and Sponsors." This guidance is intended to assist institutional review boards (IRBs), clinical investigators, and sponsors involved in clinical investigations of FDA-regulated products in carrying out their responsibilities related to informed consent.
The guidance provides the FDA’s recommendations regarding informed consent and describes regulatory requirements to help assure the protection of the rights and welfare of people participating in clinical trials. This guidance finalizes the draft guidance entitled, “Informed Consent Information Sheet: Guidance for IRBs, Clinical Investigators, and Sponsors,” issued on July 15, 2014, and supersedes FDA’s guidance entitled, “A Guide to Informed Consent,” issued in September 1998.
See the announcement for more information.
Human Subjects Office leadership is evaluating this new guidance to see if any updates are necessary to UI IRB policies, procedures and templates.

Medical Ethics Advisor Newsletter, July 2023
By Rachel Kinker, MPA

Medical Ethics Advisor (a publication of Relias, LLC) is a monthly newsletter with articles about human subjects research and medical ethics. Current and past issues of Medical Ethics Advisor and IRB Advisor are posted in the “IRB ICON Course for Researchers.” The portal to this ICON Course is on the Education and Training page of the Human Subjects Office website. This month we are spotlighting some articles about human subjects research from the July 2023 Medical Ethics Advisor Newsletter.
Ethical Approach to Informed Consent for Adults with Intellectual Disabilities
For researchers that will be working with a population with cognitive impairment, there are
additional approaches to incorporate into the informed consent process. Considerations should be made for individual differences and a variety of methods should be prepared to accom
modate the needs of each individual.
- Measure comprehension of consent and assent by developing a plan to collect feedback on whether an approach was effective or not.
- Ensure that the threshold for demonstrating capacity to consent is proportional to the complexity and risk of the study by utilizing a variety of methods for presenting information about the study.
- Use a script or checklist to guide research staff in the verbal consent process.
- Research staff should monitor for any demonstrations of dissent during the consent
- process and during study procedures.
Articles in the July 2023 Issue:
- Patients’ Race, Insurance Status Affect Likelihood of Ethics Consult
- Ethical Concerns if Study Participants Use Electronic Wearables
- Some Hospice Medical Aid in Dying Policies Require Staff to Leave Room
- The Complicated Ethics of Medical Aid in Dying
- Researchers Often Exclude Adults Living with Intellectual Disabilities
- Sham Surgeries: Should Researchers Offer the Real Procedure After the Trial?
- Ethics Consults Depend on Ability to Absorb Multiple Viewpoints
- Ethicists Debate Withdrawing ECMO Over Patient’s Objections
- Many Seriously Ill Older Adults Lack Documented Goals-of-Care Discussions

