Breadcrumb
- Home
- Get Help
- Newsletters
- September 2024 IRB Connection Newsletter
September 2024 IRB Connection Newsletter
Types of IRB Review: Exempt, Expedited, or Full Board
Herky Hints for HawkIRB: Exempt Review and Exemption Tool
Getting Credit for CITI Training at a Previous Institution
Reminder: Pre-Grant Submission Survey for Single IRB Model
Updates for the UI Research Community
In the News
IRB Educational Resources
Types of IRB Review: Exempt, Expedited, or Full Board
By Emily Shultz, CIP
At the University of Iowa (UI), an Institutional Review Board (IRB) must review and approve all human subjects research prior to the start of the research. The type of IRB review is based on the Federal Policy for the Protection of Human Subjects (known as 45 CFR 46 or the Common Rule). Any project that meets the regulatory definition of human subjects research is subject to the review and approval of the UI IRB or an external IRB.
The Common Rule outlines three types of IRB review that are based on the potential for risk to subjects. Each review type has its own unique requirements, designed to provide the guardrails for the protection of participants and the maintenance of research ethics.
In Section IV.1 of the New Project form in HawkIRB, the investigator selects the type of review that they think applies to their project. There are four options, but for the purposes of this article we will focus on:
Regular (expedited or full board) review
Exempt status
Ultimately, the Human Subjects Office (HSO) or the IRB will determine the type of review that is allowed by the regulations. The UI IRB uses the type of review with the lowest possible regulatory burden for the researcher.
Expedited review is conducted by an IRB Chair. Expedited review does not mean a quicker review process. It only means that the study meets the regulatory definition of a minimal risk study that qualifies for review by an IRB Chair or Chair Designee rather than the full IRB. Full board review means the study involves more than minimal risk to subjects and must be reviewed at a convened IRB meeting.
Depending upon the study design, level of risk to participants, and the designation indicated in the application, an IRB chair or chair designee may approve the project by the exempt or expedited review process. If the study does not meet the criteria for exempt or expedited review, the project will be referred to the convened IRB for full board review.
To be considered eligible for exempt or expedited review, the research must not be ‘greater than minimal risk’ to the participants. According to guidance from the Office of Human Research Protections (OHRP):
Exempt Review
To be reviewed at the exempt level, the project must be categorized under at least one exempt category. The IRB still considers projects in these categories to be human subjects research requiring IRB review and oversight. The project is not “exempt” from submitting an IRB application. But once the IRB grants exempt status, the research is not required to comply with the rest of the federal regulations for human subjects research.
There are eight categories of exemption in the federal regulations, however, the UI IRB has only adopted the first six of these categories:
Research conducted in established or commonly accepted educational settings, involving normal education practices.
Research that only includes interactions involving educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior (including visual or auditory recording).
Research that utilizes benign behavioral interventions in conjunction with collection of information from adults through verbal or written responses (including data entry) or audiovisual recording.
Research involving secondary uses of identifiable private information and/or identifiable biospecimens.
Research or demonstration project conducted or supported by a federal department or agency or otherwise subject to approval by the conducting or supporting department or agency's head or delegate.
Research on taste and food quality evaluation or a consumer acceptance study.
Note: if minors are included in the recruitment pool, exempt categories 2 and 3 cannot be applied and the project will need to be reviewed at the expedited or full board level. At the University of Iowa, the IRB Chair or designee is the sole authority for determining whether the research meets the exempt criteria.
If the study is minimal risk but does not fall under one of the exempt categories, the IRB Chair may elect to review the project using expedited review procedures.
Expedited Review
A single IRB chair, rather than the full convened board, can approve research that qualifies for expedited review. Contrary to the definition and common usage of the word “expedited,” this type of review is not necessarily faster. Based on the HawkIRB application, the IRB chair determines whether the research meets one or more of the expedited review criteria. Even when the project appears to meet the criteria for expedited review, the IRB chair may refer the application for full board review if they have any concerns about approval of the project. An IRB chair can approve a project using expedited review procedures, but they cannot disapprove a project.
An IRB chair or designee can also use expedited review of modifications to previously approved research projects if the modification involves only minor changes to the approved project. For projects that were initially reviewed by the full board, the IRB can use expedited review procedures for modifications that are considered minimal risk. The IRB can also use expedited review procedures for the continuing review of greater than minimal risk research under the following circumstances:
No subjects have been enrolled and no additional risks have been identified, OR
The research is permanently closed to enrollment,
All subjects have completed all research-related interventions, AND
The HawkIRB application remains open only for:
a. Long-term follow up of subjects, OR
b. Data analysis
Full Board Review
The full IRB must review human subjects research that is greater than minimal risk for participants at a convened meeting. A research study undergoes full board review after the IRB chair, or their designee, reviews the entire application and determines that the project constitutes human subjects research which is greater than minimal risk.
Full board review is required in the following instances:
The study involves investigational drugs, devices, or biologics that require an Investigational New Drug (IND) or Investigational Drug Exemption (IDE) from the FDA
Continuing review for studies that were initially reviewed by the full board (except when the board has determined that there is no more than minimal risk and no additional risks have been identified)
When there is greater than minimal risk of criminal or civil liability to subjects
When participation in the research poses a greater than minimal risk to the subject as it may be damaging to subjects financial standing employability, insurability, reputation or be stigmatizing (unless appropriate protections are implemented)
Classified research involving human subjects
The study needs a Non-Significant Risk Determination because it is a Non-Significant Risk device
When there is a Conflict of Interest that needs to be managed
At individual IRB chair discretion
Summary
The categories of review allow the IRB to assess the potential for risk to the human subjects and to provide the appropriate level of oversight as proscribed by federal regulations and institutional policies. Although there are sometimes nuances involved in assessing the category of review, the driving force behind the assessment is the ethical conduct of research and the protection of participants.
Questions about this topic? You can email the HSO Education and Outreach team, or come to Office Hours to speak directly to a member of the HSO team.
Have an idea for an IRB Connection newsletter article? Let us know!
Herky Hints for HawkIRB: Exempt Review and Exemption Tool
By Rachel Kinker, MPH
An Institutional Review Board (IRB) must review and approve research projects that meet the regulatory definition of human subjects research. Regulatory requirements charge the IRB with determining the category of review.
Some research may qualify for exemption from certain federal regulations: 45 CFR 46.104 (HHS) and 21 CFR 56.104 (FDA). At the University of Iowa, an IRB chair designee can determine whether a research project meets the exempt criteria based on review and approval of an exempt new project application in HawkIRB. (For more information about categories of review, see Types of Review in this month’s IRB Connection newsletter.)
In HawkIRB, the new project form for exempt status has approximately one hundred fewer questions than a project under regular review. When the PI selects “Exempt review” in Section IV.1, the application changes to collect only the information an IRB chair designee will need to determine what category(ies) of exempt status apply.
When “Exempt review” is selected, Sections IV.1. a and b will open. Section IV.1.a pertains to the study design and subject population. Responses to this question will indicate whether the project is eligible for exempt review.
For certain subject populations or study designs, a hard stop will appear in HawkIRB indicating that the project is not eligible for Exempt status and needs to go through regular review.
The message will appear if any of the following are selected:
Study involves prisoners as subjects
Study involves enrolling cognitively impaired subjects
Study involves collecting or retaining a Social Security Number (SSN)
Study involves use of the Iowa City VA Health Care System (VAHCS)
Study involves review by MRPC (Medical Radiation Protection Committee), P&T (Pharmacy and Therapeutics Committee), or IBC (Institutional Biosafety Committee)
Study involves human subjects research where DHHS (Department of Health and Human Services) has prohibited the exemption
Additionally, if minors are indicated as part of the study population and the selection “Study involves deception” is made, the hard stop message will also appear and prompt the selection of “regular review” in Section IV.1. The selections in IV.1.a determine what exemption categories can be selected in Section IV.1.b. Any categories that are no longer available will be greyed out. For example, for studies involving deception, “Category 3” is the only option for exempt review.
Based on the exemption category(ies) selected in Section IV.1.b, HawkIRB will open additional questions.
If the study will include a consent process, the investigator may select “Exempt Information Sheet” from the dropdown menu in the Attachments section of HawkIRB, instead of utilizing a full informed consent document.

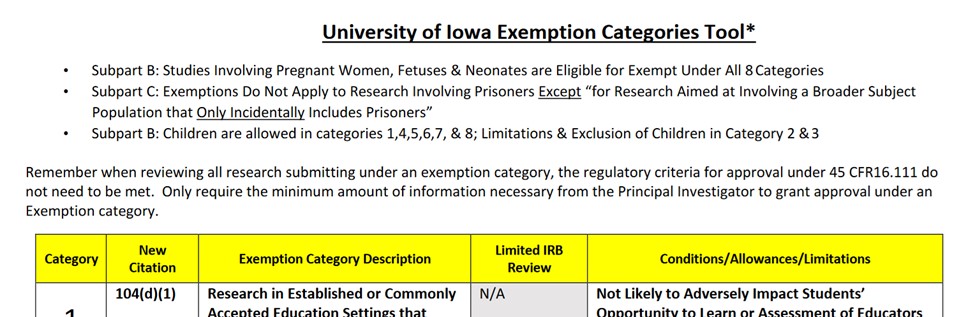
For assistance with selecting the correct category of exemption, the Exemption Tool is available on the HSO website on the Educational Tool page.
Questions about this topic? Email the HSO Education and Outreach team or come to Office Hours to speak directly to a member of the HSO team.
Have an idea for a future Herky Hint for HawkIRB? Let us know!
Getting Credit for CITI Training at a Previous Institution
All investigators conducting human subjects research at the University of Iowa (UI) or at the Iowa City VA Health Care System (VAHCS) are required to complete an education program and become "certified" in human subject protections through the Collaborative Institutional Training Initiative (CITI) program. Many investigators will come to the UI having previously completed some form of CITI training.
Each institution establishes modules that meet their requirements for certification. Because the training modules in the CITI online course are institution-specific, the University of Iowa cannot simply accept a CITI completion certificate from another institution. However, if a researcher has completed modules that are also a part of the UI training requirement, they can receive credit for them.
To receive credit for previous modules and complete the University of Iowa CITI course modules, the investigator will need to update their CITI program profile to affiliate with the UI. To affiliate with the UI, in the ‘Select Your Organization Affiliation’ section, type in ‘University of Iowa.’ Please see the complete CITI instructions: How to add/change your affiliated institution or transfer completions.
The University of Iowa requires the following modules for human subjects protection training.
For IRB-01 Biomedical, the required modules are:
Introduction
History and Ethical Principles
Basic Institutional Review Board (IRB) Regulations and Review Process
Informed Consent
Social and Behavioral Research for Biomedical Researchers
Records-Based Research
Genetics Research in Human Populations
Research with Protected Populations - Vulnerable Subjects: An Overview
University of Iowa
For IRB-02 Social & Behavioral, the required modules are:
Introduction
History and Ethical Principles - SBR
Defining Research with Human Subjects - SBR
The Regulations and the Social and Behavioral Sciences - SBR
Assessing Risk in Social and Behavioral Sciences - SBR
Informed Consent - SBR
Privacy & Confidentiality - SBR
University of Iowa
Researchers typically complete Group 1 (biomedical) or Group 2 (social/behavioral) training based on the college or department in which they conduct research. Regardless of their college or department, researchers conducting biomedical research (i.e. specimen collection, new drug applications, etc.) should complete the Group 1 training.
Researchers who completed human subjects protection training at a previous institution and have affiliated with the UI, once enrolled in the course (IRB-01 or IRB-02), the CITI program will indicate which UI-required modules are incomplete. Once the researcher completes these modules, the CITI Program will notify the UI IRB that training is complete, and we will manually add the researcher to the Certified Investigator database in HawkIRB.
Additional information about completing the UI CITI training is available on the HSO website: Human Subjects Protection Training from Another Institution and the UI CITI User’s Guide on the HSO website for step-by-step instructions. If you have any questions regarding this process, please contact us at irb-outreach@uiowa.edu.
Reminder: Pre-Grant Submission Survey for Single IRB Model
Federal agencies require the use of a single IRB (sIRB) for federally funded research conducted at multiple sites. This means one IRB oversees research conducted at some or all of the study sites. The budget must include the fees for this type of IRB review and this type of research may require some additional approvals and agreements. Researchers can access information about UI IRB fees for budget planning.
If you are planning to submit a grant proposal to the National Institutes of Health (NIH) or any other federal agency that requires the sIRB model, complete the Pre-Grant Submission Survey as soon as you become aware of the award notice. It is best to complete this survey well in advance, especially if the UI IRB is serving as the lead IRB over non-UI sites or relying on an external IRB as an expectation of the grant.
If you have questions or need any assistance, contact the External IRB Team at uirb-external@uiowa.edu.
