Breadcrumb
- Home
- Get Help
- Newsletters
- November 2022 IRB Connection Newsletter
November 2022 IRB Connection Newsletter
IRB Presentation Recap: Checklists and Guidance Documents Galore!
NIH-Funded Clinical Trials: Compliance with Reporting Results
From WIRB to WCG, Website Updates, and More
Food and Drug Administration Proposed Rule Change and Draft Guidance
Medical Ethics Advisor Newsletter, October 2022
In the News
IRB Presentation Recap: Checklists and Guidance Documents Galore!
By Kelly O’Berry, BS, CIP

On October 5, 2022, The Human Subjects Office shared information and showed how to use eleven checklists and guidance documents available for the UI research community. One is brand new! A few of them were recently posted on the Human Subjects Office website. For additional information, view the recorded presentation in the IRB ICON Course for Researchers and the Checklists and Guidance Documents Educational Tool.
NIH-Funded Clinical Trials: Compliance with Reporting Results
By Fozia Ghafoor, MBBS

In August 2022, the Office of Inspector General (OIG) published a report of audit findings regarding clinical trial results reporting. Results for the majority of NIH-funded clinical trials completed in 2018 were either late or not submitted at all in ClinicalTrials.gov. The objective of the audit was to determine whether the National Institutes of Health (NIH) ensured that NIH-funded clinical trials complied with Federal reporting requirements. Based on the OIG recommendations, NIH started sending letters of noncompliance to the investigators who failed to report results by the required deadline.
NIH provides two types of funding:
- Intramural - for clinical trials carried out by NIH scientists in NIH laboratories on its campuses
- Extramural - awards to the community of scientists at universities, medical centers, hospitals, and research institutions throughout the United States and abroad
NIH must ensure that the results of NIH-funded Intramural and Extramural clinical trials are reported on ClinicalTrials.gov, a publicly accessible registry and results database of publicly and privately supported clinical studies of human participants. Posted results provide information to the public for understanding the safety and effectiveness of different interventions.
The OIG reviewed 72 NIH-funded clinical trials that were expected by Federal law and NIH policy to submit results in 2019 or 2020. Auditors compared the due date for the results with the date results were actually submitted to ascertain compliance with reporting requirements. OIG also checked to see if NIH evaluated and posted clinical trial results provided by the relevant parties on ClinicalTrials.gov within the required 30 days.
The audit concluded that NIH did not ensure compliance with federal reporting requirements for responsible parties to submit clinical trial findings to ClinicalTrials.gov. The table below shows the number of clinical trials reviewed in the audit with due date submissions in 2019 or 2020 that were submitted on time, late, or not submitted at all.
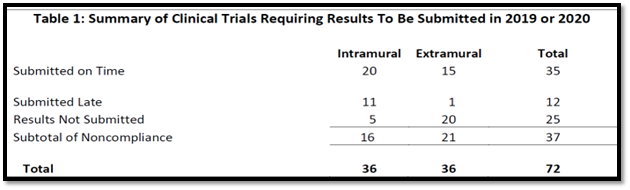
Of the 72 NIH-funded clinical trials that OIG reviewed,
- Responsible parties associated with 35 clinical trials (20 Intramural-funded and 15 Extramural-funded) complied with Federal reporting requirements
- Responsible parties associated with 37 other clinical trials (16 Intramural-funded and 21 Extramural-funded) did not comply with Federal reporting requirements. They either:
- did not submit their results (5 Intramural-funded and 20 Extramural-funded)
- or submitted them late (11 Intramural-funded and 1 Extramural-funded)
- NIH complied with the Federal reporting requirement to post clinical trial results to ClinicalTrials.gov within 30 days of the submission date for the47 NIH-funded clinical trials in which responsible parties submitted their results (35 submitted on time and 12 submitted late). NIH posted these results in an average of 14 days. NIH was not able to post the results of the remaining 25 clinical trials because the results were not submitted by the responsible parties.
Although NIH has a policy requiring submission of results from all NIH-funded clinical trials and the investigator or awardee performing the obligations of a responsible party for all NIH-funded clinical trials, OIG found that NIH did not take necessary actions for ensuring that responsible parties submitted the results of clinical trials, took limited enforcement action when there was an incident of noncompliance, and continued to fund additional research for responsible parties that did not submit the results of completed clinical trials.
OIG recommended that NIH:
- improve procedures to ensure compliance with requirements to submit results to ClinicalTrials.gov in a timely manner,
- take enforcement actions against responsible parties that are late in submitting trial results or do not submit results, and
- work with the responsible parties to understand their challenges related to ClinicalTrials.gov and implement procedures to address the challenges.
NIH agreed with the OIG recommendations and implemented the following changes
- Develop a centralized workflow for verifying extramural compliance
- Introduce new forms to enable better tracking
- Integrate electronic systems to facilitate non-compliance alerts
- Validate and take compliance action prior to issuing subsequent awards
- Strengthen compliance and enforcement procedures
- Establish a forum for responsible parties to provide user experience to NIH. NIH regularly conducts training and outreach to educate investigators, administrators, and staff on clinicaltrials.gov registration and reporting requirements.
- Redesign Clinicaltrials.gov to improve the user interface based on extensive usability studies.
NIH started sending letters of noncompliance to those who have failed to report results by the required deadline. Investigators have 4 weeks to provide evidence that they either submitted the results on ClinicalTrials.gov or that result information is not required at this time.
UI investigators with an NIH-funded clinical trial who receive a notice of noncompliance should immediately notify the UI PRS Administrator (ct-gov@uiowa.edu or (319)335-6564).
From WIRB to WCG, Website Updates, and More
Mayzie Tucker, BBA
In 2020, WIRB integrated with four independent IRBs and is now operating under the title, WCG (WIRB-Copernicus Group). In response to this change, the Human Subjects Office (HSO) updated the WCG Guidance webpage to promote readability and update current guidance, in addition to reflecting the updated operating title for this commercial IRB.

After WIRB (Western IRB) integrated with four independent IRBs — Copernicus Group IRB, New England IRB, Aspire IRB, and Midlands IRB – the new operating title for the organization is now WCG (WIRB-Copernicus Group). With change, comes new guidance and processes for the UI External IRB Program and the UI research community.
WCG also integrated a new electronic application platform in early 2021, called WCG IRB Connexus. This system is fully operational and should be used for all future research study submissions utilizing WCG as the IRB of Record. The WCG Guidance web page includes references to this eResearch platform.
WCG Webpage Updates
The WCG webpage also has several other content updates:
- Order of events for the HawkIRB and WCG Submission Process
- Guidance regarding Post-Approval Submissions and Fees across all studies relying on an External IRB (Modification, CRs, Closures, and REFs)
- Improved readability throughout
We hope the updated guidance provides a good reference point to the researchers utilizing WCG as an IRB of Record. If you have questions regarding the updated content, please contact Mayzie Tucker, Commercial IRB Coordinator, by email at uiwirb@uiowa.edu or by phone at (319) 467-4182.
Food and Drug Administration Proposed Rule Change and Draft Guidance
By Kelly O’Berry, BS, CIP

This fall, the U.S. Food and Drug Administration (FDA) released two significant pieces of guidance related to human subjects research.
Human Subjects Research
On September 28, 2022, the FDA opened the comment period for a proposed rule to amend the regulations for the Protection of Human subjects and Institutional Review Boards. The proposed amendments will harmonize some sections of FDA regulations with the Department of Health and Human Services revised Common Rule that took effect in January 2019. These amendments are proposed to fulfill the requirements of the 21st Century Cures Act and are intended to reduce regulatory burden on IRBs, sponsors and investigators.
The revised regulations amend parts of 21 CFR 50 and 21 CFR 56 to:
- Revise content, organization, and presentation of information in the Informed Consent Document
- Add new basic and additional elements of informed consent
- Add a provision to allow IRBs to eliminate the continuing review of some research
- Revise IRB recordkeeping requirements related to continuing review
- Add or modify some definitions
There is also a proposed revision to 21 CFR 812 related to progress reports, in conjunction with the proposed changes to 21 CFR 56 regarding continuing review.
The comment period for written or electronic comments is open through November 28, 2022.
Expanded Access
On November 1, 2022, the FDA issued revised draft guidance for Expanded Access to Investigational Drugs for Treatment use Questions and Answers. This includes answers to frequently asked questions the FDA received since the current guidance by the same name was last updated in 2017. The new guidance also includes recommendations for fulfilling the requirements of the 21st Century Cures Act and the FDA Reauthorization Act of 2017 related to expanded access. There are significant changes related to:
- IRB review
- Informed consent
- Sponsors having publicly available policies for evaluating and responding to expanded access requests
The comment period for written or electronic comments is open through January 3, 2023.
Medical Ethics Advisor Newsletter, October 2022
By Rachel Kinker, MPA

Medical Ethics Advisor (a publication of Relias, LLC) is a monthly newsletter with articles about human subjects research and medical ethics. Current and past issues of Medical Ethics Advisor and IRB Advisor are posted in the “IRB ICON Course for Researchers.” The portal to this ICON Course is on the Education and Training page of the Human Subjects Office website. This month we are spotlighting some articles about human subjects research from the October 2022 Medical Ethics Advisor Newsletter.
Some IRBs Expand Their Purview to Consider Scientific Merit
In some instances, IRBs offer detailed advice on study design, methodological advice, technical aspects, and scientific merit, expanding their purview beyond the expected review of participant safety and ethics. These additional considerations can result in protocol deferrals. The most common ethics related reasons for protocol deferral include;
- Inadequate informed consent
- Insufficient protection of participants safety
- Inadequate detail of risk assessment
- Inadequate minimization of risks
Consideration for investigators to avoid delays and/or protocol deferrals:
- Fill out IRB forms correctly and follow all instructions
- Less experienced researchers should consider working with more experienced researchers to submit IRB protocols
- Pay special attention to providing adequate detail to enable reviewers to make determinations regarding study safety
- Ensure there is consistency between the IRB protocol and the associated consent form
Articles in the October 2022 Issue:
- Fraud Allegations Involving Alzheimer’s Disease Study Raise Concerns
- Ethicists Asked to Weigh in on Medical Necessity of Abortion
- New Requirements Are Discouraging Physicians from Writing DNR Orders
- Court-Appointed Guardians for Unrepresented Patients
- Making Ethical Decisions on Genetic Testing, Precision Medicine
- Ethicists Strive to Make Training for Consults More Consistent
- Encourage Reluctant Clinicians to Contact Ethics
- Updated Recommendations on Pediatric End-of Life Care
- Nurses and Physicians Find Ethics Consults Helpful, But for Different Reasons

In the News, November 2022

- Expanded Access for Tecovirimat (TPOXX): An Explainer for IRB and other regulatory professionals, Ampersand, the PRIM&R blog
- Critical role IRBs could play in shaping health outcomes in minority groups, Ampersand, the PRIM&R blog
- FDA releases draft guidance on ethical consideration for pediatric clinical investigations, Ampersand, the PRIM&R blog
- The White House’s ‘AI Bill of Rights’ outlines five principles to make artificial intelligence safer, more transparent and less discriminatory, The Conversation
- A blood test that screens for multiple cancers at once promises to boost early detection, The Conversation
- One of the Biggest Problems in Biology Has Finally Been Solved, Scientific American