Breadcrumb
- Home
- Get Help
- Newsletters
- May 2024 IRB Connection Newsletter
May 2024 IRB Connection Newsletter
Non-UI Team Members Human Subjects Protection Training Requirements
Herky Hint: Before a PI Leaves the UI
AAHRPP Accreditation Review – Domain II. IRB
Reminder: Complete the Pre-Grant Submission Survey for Single IRB Model
New Glossary provides Lay Language for Clinical Trials
For Researchers Traveling Abroad
In the News
Non-UI Team Members Human Subjects Protection Training Requirements
By Rachel Kinker, MPA
All members of a research team conducting human subjects research at the UI or Iowa City Veterans Affairs Healthcare System are required to complete human subjects protection training through the Collaborative Institutional Training Initiative Program (CITI). This training is required to ensure that all research team members understand the ethical principles of working with human research subjects and the regulatory requirements for conducting research.
If a study has non-UI team members from an institution without an IRB, they must complete CITI training. If the study team includes non-UI team members from the community (not affiliated with an academic institution with an IRB), they will need to complete CITI training, unless the PI consults with the Human Subjects Office about alternative approaches to training.
Non-UI team members at an institution with an IRB that is relying on the UI IRB will complete their own institution’s training requirement. Reliance agreements between the UI and other institutions will indicate the roles and responsibilities for relying sites, typically the relying site is responsible for tracking the completion of the Human Subjects Protection training for their members.
Best Practices for Adding a Non-UI Team Member
If the non-UI Team member needs to complete the UI training requirement, after completing the training they should watch for an email from irb-certifications@uiowa.edu requesting the following information:
Individual’s name
Institution they are affiliated with (if applicable)
Name of the UI PI of the project they will be associated with
HawkIRB project number
Having this information will allow the review team and CITI administrator to get the individual added to the UI Certified Investigator Database. The non-UI team member cannot be added until the information has been added to their profile in the UI Certified Investigator Database. Alternatively, the PI or a research team member can send an email to irb-certifications@uiowa.edu with names of non-UI team members and the information requested above.
More information about summer research internship team members is available in the May 2023 IRB Connection article ‘Summer Student Research Internships article. For additional guidance on when you must add a non-UI team member to the research team, please see the September 2023 IRB Connection article ‘External IRB Q&A: Not UI Team Members’.
Please reach out to irb-certifications@uiowa.edu with any questions.
Herky Hint: Before a PI Leaves the UI
By Rachel Kinker, MPA
Before a PI leaves the UI, they will need to inform the IRB and make changes to their project information in HawkIRB. The changes required will depend on several factors.
These may include:
closing the project in HawkIRB; or
naming a new PI for the project in HawkIRB;
complying with the IRB record retention policy; and
completing a data use agreement, as appropriate.
Planning Ahead for HawkIRB Access
When a PI leaves the UI, they will no longer have access to the HawkIRB system and are considered “deactivated” once their UI appointment has ended. This status is based on their UI employment status (HR records) or enrollment status (registrar records). If the PI has HawkIRB delegates, the delegates can complete limited submissions once the PI is deactivated, but only for the projects to which they have been assigned as a delegate. These submissions include submitting Project Close forms and Modification forms.
As soon as the departure date is known, the PI should submit the appropriate forms to the IRB and others in the Human Research Protection Program (HRPP). These are a Project Closure Form (if the research will no longer be conducted at the UI) or a Modification Form to change the PI (if the study is not complete and research activity will continue at the UI under a new PI).
Research Results
The UI generally owns the primary research results generated from research conducted under its jurisdiction (see the Research Data Policy for more information). When a PI plans to leave the UI and would like to take the original data to a new institution, the transfer of the data/samples to the new institution must follow established guidelines. See Section II, Part 24 of the UI IRB Standard Operating Procedures and Researcher Guide and information about Data Use Agreements on the Division of Sponsored Programs website.
Records Retention
While the study is open and for a specified period after the study has closed, the University/VA Healthcare System and federal regulations require that the PI maintains the following records:
Copies of human subjects application forms
Notices of approval
Signed Informed Consent Documents
Prior to departure, the PI should save copies of the completed application and IRB approval memos before they lose access to HawkIRB. All original, signed Informed Consent Documents must remain on the UI campus for the required time:
For studies that do not involve Protected Health Information (PHI), records must be maintained for at least three years following the close do the study in HawkIRB
For studies that do involve PHI, records must be maintained for at least six years after the close of the study in HawkIRB
The UI IRB record retention policy only applies to the documents listed above and not the data collected for the study. Study data must be stored according to confidentiality protections outlined in Section X of the HawkIRB application. Please note that study sponsors may require a longer retention period and/or broader definition of the records that need to be maintained.
Ultimately, the PI is responsible for maintaining accurate files of IRB correspondence, approvals, and research records during and after completion of the study. Please see the UI IRB Standard Operating Procedure and Researcher Guide, Sec. II, Part 23 for additional details.
To Submit a Project Close Form:
When data collection is complete and the PI/research team will not work with identifiable research data/samples, they should submit a project closure. If the PI is planning to take data to the new institution, they should make sure that it is described in the HawkIRB application and approved by the IRB prior to closure.
To create a Project Close form, log into your HawkIRB inbox. Under ‘Projects,’ click on the IRB number of the study you would like to close.
Under ‘Create Form,’ generate a Project Close Form.
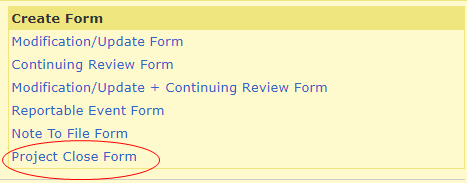
The project will close as soon as the form is submitted. Once the form is submitted, the research team may no longer collect data about any of the subjects in the study, contact the subjects for research purposes, or work with identified data/samples.
If you will be collecting follow-up data the project should remain open, even if new subjects are not being enrolled. See the UI IRB Standard Operating Procedures and Researcher Guide Sec. II, Part 22
Projects should not be closed unless all the following have been completed:
Protocol-indicated research activities, including interaction with subjects and collection of data or specimens
Collection of data about subjects even when no subject contact is necessary
‘Cleaning’ of data
Analysis of identified or coded data for research purposes or during the publication process
Any other research use of data that involves access to identified or coded data or specimens collected during the conduct of research
Submit a Modification Form:
The PI should submit a Modification form if (1) the study is not complete, and a new PI will be named on the application and/or (2) to request IRB approval if the PI will take research data/samples to a new institution or plans to have continued access after departure.
If the PI will take identifiable research data/samples:
Contact the Division of Sponsored Programs (DSP) to determine if a Data Use Agreement or Material Transfer Agreement is needed
Obtain approval for the transfer of data/samples from the PIs department
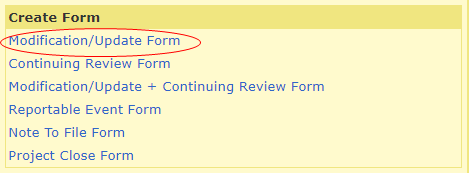
To create a Modification form, log into your HawkIRB inbox. Under ‘Projects,’ click on the IRB number of the study you would like to modify.
Under ‘Create Form’ click on the Modification/Update Form
After starting the modification, you will be routed to the Frequent Mods portion of the Modification Index. If you will be sharing data, please see the November 2023 IRB Connection article ‘Best Practices for Research Collaboration and Data Sharing’.
To change the PI, select the option ‘Change the Principal Investigator.’ Enter the information of the new PI and select the ‘Set Principal Investigator’ button and then save the form.
To share or take data from the UI, modify section X.5 and 6, please see the article November 2023 IRB Connection article ‘Herky Hint: What to do with all that Data? Data Sharing and Security Guidelines’
Additional Information about Transfer/Departure/Change of Status Forms and Procedures is also available in this April 2023 article.
AAHRPP Accreditation Review – Domain II. IRB
By Emily Shultz, CIP
The University of Iowa Human Research Protection Program (HRPP) is in the process of applying for reaccreditation to the Association for the Accreditation of Human Research Protection Programs (AAHRPP). This article is part of a series outlining the certification process and requirements.
In previous articles, we introduced the three domains of the AAHRP accreditation review:
The organization
The institutional review board or ethics committee
Researchers and research staff members
This month we will focus on the IRB Domain. This area of the application and review process is focused on the requirements for the ethical oversight of research by an institutional review board (IRB) or ethics committee (EC). The supporting documents submitted with the AAHRPP application for this Domain will include HawkIRB application forms, workflow communications, and reviewer checklists; as well as meeting materials, internal standard operating procedures (SOPs) and policies in the UI Standard Operating Procedures and Researcher Guide.
This article outlines the five Standards under the IRB Domain and the specific supporting documents for each standard
The federal regulations have specific requirements for the types of expertise and experience the board members must possess (e.g. scientific, non-scientific, and community/non-affiliated members). Additionally, the institution must manage potential conflicts of interest. To show that the UI IRB meets these requirements, the AAHRPP application will provide supporting documents, including the IRB rosters, disclosure forms, meeting agendas and meeting minutes.
The nine Elements under this Standard provide an outline for assessing whether the IRB members and staff participating in the review or research projects have protected the human participants utilizing the appropriate expertise, category of review (e.g., full board, expedited, limited review, or exempt), and the criteria for approval. The supporting documents for these Elements will include the HawkIRB application forms, reviewer checklists, and template letters to researchers.
This Standard addresses whether the IRB follows written policies and procedures for the approval of research. When assessing the application for this Standard, AAHRPP reviewers will rely on supporting documents that include the HawkIRB application forms, the primary reviewer checklists and the consent templates.
Focusing on how the IRB ensures protections for vulnerable populations, the supporting documents for this Standard will include HawkIRB application forms, reviewer checklists and informed consent templates.
This Standard contains two elements which address that the IRB has and follows policies and procedures for maintaining documents as proscribed by regulation and policies. The supporting documentation for the application under this Standard will include SOPs, HawkIRB, the IRB meeting minutes.
In our next installment about AAHRP Accreditation, we will focus on the next Domain: Researchers and research staff members.
The information for this article was adapted from the AAHRPP website.
Reminder: Complete the Pre-Grant Submission Survey for Single IRB Model
Federal agencies require the use of a single IRB (sIRB) for federally funded research conducted at multiple sites. This means one IRB oversees research conducted at some or all study sites. The budget must include the fees for this type of IRB review, and additional approvals and agreements may be required for this type of research. Researchers can access information about UI IRB fees for budget planning.
For grant proposals to the National Institutes of Health (NIH) or any other federal agency that requires the sIRB model, complete the Pre-Grant Submission Survey prior to grant submission. Plan ahead and complete this survey well in advance. In some situations, the UI IRB cannot, or will not, serve as the lead IRB (which will impact your study’s sIRB plan or budget).
If you have questions or need any assistance, contact the External IRB Team at uirb-external@uiowa.edu
New Glossary provides Lay Language for Clinical Trials
By Kelly O’Berry, BS, CIP
A new Clinical Research Glossary is available to help describe research procedures in a language that potential subjects can understand. The Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women’s Hospital and Harvard, has developed the glossary to help research participants make better informed decisions about whether to participate in a research study.
The Glossary contains definitions, examples, and images associated with each word or concept. The content and images are free to use under the MRCT Center Creative Commons License. The Accessibility Menu button on each page ensures that this resource is widely usable. It can be used to translate the Glossary into 50+ languages.
For Researchers Traveling Abroad
Leslie Revaux, director of strategic communications, posted an article on the Office of Vice President for Research (VPR) website April 29, 2024, titled “Traveling abroad for presentations or research this summer?”
The article provides information and links to a wide range of resources available to support international collaboration, including:
Division of Sponsored Programs
Conflict of Interest in Research Office
Information Technology
Export control coordinators
Additional information for researchers who will be conducting research outside the United States is also available in a previous IRB Connection newsletter from April 2022: Going Abroad? Plan Ahead to Conduct Research Outside the U.S.
In the News, May 2024
