Breadcrumb
- Home
- Get Help
- Newsletters
- February 2025 IRB Connection Newsletter
February 2025 IRB Connection Newsletter
Understanding Data Use Agreements and Material Transfer Agreements in Sponsored Research
Herky Hints for HawkIRB: Data Sharing
Understanding Data Use Agreements and Material Transfer Agreements in Sponsored Research
By Jessica Boyle, Associate Director, Division of Sponsored Programs
Data use agreements (DUAs) and material transfer agreements (MTAs) play a crucial role in facilitating collaboration while safeguarding intellectual property and ensuring compliance with institutional, ethical, and legal standards, DUAs and MTAs serve distinct purposes and involve different considerations.
What Are Data Use Agreements (DUAs)?
A DUA is a legal document that governs the transfer of data between two entities for research purposes. Typically, DUAs are necessary when data is shared between a data provider (e.g., a research institution, government agency, or private organization) and a data recipient (e.g., a university or researcher). DUAs provide an outline of:
The type of data being shared, including whether it includes sensitive or personally identifiable information (PII).
The purposes for which the data may be used.
Restrictions on how the data can be accessed, stored, or shared further.
Requirements for safeguarding the data, including compliance with laws such as the Health Insurance Portability and Accountability Act (HIPAA) or the General Data Protection Regulation (GDPR).
Intellectual property rights associated with derivative works or findings.
DUAs are particularly important for ensuring the ethical use of data, protecting participants' privacy, and maintaining the integrity of research.
What Are Material Transfer Agreements (MTAs)?
MTAs are legal contracts that govern the transfer of tangible research materials—such as biological samples, chemical compounds—from one party to another. MTAs are essential when one institution provides materials to another for research purposes. MTAs cover:
The description and intended use of the materials.
Ownership of the materials and derivatives created during research.
Liability and indemnification clauses related to the use of the materials.
Confidentiality and intellectual property provisions.
MTAs ensure that proprietary materials are used responsibly, that the provider’s rights are respected, and that the recipient adheres to agreed-upon research objectives.
How DUAs and MTAs Support Research
Both DUAs and MTAs foster collaborative research efforts while addressing potential risks:
Facilitating Collaboration: DUAs and MTAs enable researchers to access valuable resources—be it datasets or materials—that might otherwise be unavailable. This access enhances research capabilities and accelerates scientific discovery.
Protecting Intellectual Property: Both agreements ensure that proprietary rights and contributions of the provider are acknowledged and safeguarded. This is crucial for fostering trust and encouraging the sharing of resources.
Ensuring Compliance: DUAs and MTAs establish clear guidelines to ensure compliance with legal, ethical, and institutional policies, reducing the likelihood of disputes or regulatory violations.
Mitigating Risks: These agreements address potential liabilities, whether related to the misuse of sensitive data or hazardous materials, thereby protecting all parties involved.
Challenges with DUAs and MTAs
While DUAs and MTAs are beneficial, they also present challenges:
Administrative Complexity: Drafting, negotiating, and executing DUAs and MTAs can be time-consuming, especially in institutions with high volumes of collaborative research. Variability in terms and conditions across institutions can further complicate negotiations.
Balancing Interests: Providers may impose restrictive terms to protect their assets, which could limit the recipient’s ability to use the data or materials effectively. Striking a balance between protecting the provider’s interests and ensuring research flexibility is a common challenge.
Regulatory Compliance: Navigating complex legal frameworks—such as export control laws, human subjects’ protections, privacy regulations, and biosafety standards—can be daunting. Failure to address these issues adequately can lead to compliance violations.
Data Sensitivity and Security: For DUAs, ensuring the secure handling of sensitive data, especially when dealing with PII or proprietary datasets, requires robust infrastructure and training. Breaches can have severe legal and reputational consequences.
Enforcement and Monitoring: Institutions often lack resources to monitor compliance with the terms of DUAs and MTAs, leaving them vulnerable to misuse or unauthorized dissemination of resources.
Conclusion
Data use agreements and material transfer agreements facilitate the exchange of data and materials while protecting the interests of all parties involved and ensuring compliance with applicable regulations. Implementation of these agreements include challenges that require careful negotiation, institutional support, and ongoing monitoring. In addressing these challenges effectively, institutions enhance their research capabilities and contribute to advancing knowledge across disciplines.
Questions? Contact the Division of Sponsored Programs at (319) 335-2123 or dsp-contracts@uiowa.edu
By Emily Shultz, CIP
When collaborating with other researchers or institutions, the requirements for data sharing include IRB approval, subject consent, and possibly, a data use agreement between the UI and the institution of the recipient.
HawkIRB application:
In the HawkIRB application, the information provided in section X.4 will need to address the data security plan or confidentiality protections for transmission or transport of the data or specimens. Make sure to work with the departmental IT representative to establish an appropriate plan, depending on the sensitivity of the data/specimens.
In section X.5, describe data sharing in the question about whether study data is only accessible to members of the UI research team. When you share data with a non-UI colleague, or if research team members leave the UI and want to take data with them, the appropriate answer to section X.5 is “No.”

This response will trigger question X.6, in which you describe the individual and/or entity with whom you will share data/specimens. Specify all individuals who are not members of the UI research team that may have access to data/specimens or with whom they will be shared. Indicate the name of their institution. Describe how they will have access to the data/specimens and whether they will be identifiable, coded, or de-identified.
Either specify in section X.6 that you will work with the Division of Sponsored Programs (DSP) to establish a data use agreement (DUA) or go ahead and initiate the process with DSP and attach a copy of the initiated DUA in the miscellaneous attachment category. That demonstrates to the IRB that you know this is a necessary step in the process.
If you know up front that you will share data or specimens outside the UI, you can describe plans for that in the sections described above in the new project form. If you decide to share data/specimens outside the UI after you have IRB approval for the project, submit a modification form. If the UI IRB approves a data sharing plan in a modification form, the research team may only share data/samples that were collected after the approval date or reconsent enrolled subjects on the new version of the consent document that describes data sharing.
Future Research
Section XII of the new project application asks questions about participant data being kept specifically for future research. The information provided in response to questions XII.1 through XII.3 is specific to data being kept for the purpose of recontacting participants.
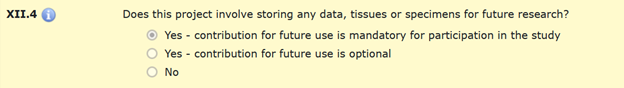
The response to question XII.4 is specific to keeping data, tissues or specimens for future research.
The information specified in section XII will need to be explained to participants in the consent process.
Informed Consent
The informed consent process must include information about data sharing in the section, Tissue/Blood/Data Storage for Future Use. Use the template to describe for participants in lay language:
which of their data will be shared and with whom,
whether there will be identifiers attached to the data,
where the data will be stored, and
provide the participant with the option to agree or disagree to participate in data sharing.
If you add data sharing after initial IRB approval, use the edit function to revise the consent document. Generate a new IRB consent template and then copy and paste the template language into the previously approved consent document.
Other Considerations
If you collaborate with another institution you may need to contact the external IRB coordinator. They will help you establish the appropriate agreements to extend UI IRB oversight to an individual or institution/agency.
It is also important to consider, depending upon the purpose or reason for the data sharing, additional information may also need to be added to section VII.E.6., where study procedures are explained.
Also keep in mind, it is considered ‘data sharing’ when the principal investigator or a research team member leaves the UI and wants to take data/specimens with them.
For more information about data security and data agreements, see this month’s article by Jessica Boyle, Division of Sponsored Programs, and additional information in the November 2023 IRB Connection Newsletter.