Breadcrumb
- Home
- Get Help
- Newsletters
- April 2025 IRB Connection Newsletter
April 2025 IRB Connection Newsletter
Providing Large Media Files for IRB Review
Before a PI Leaves the UI
When submitting materials for review by the IRB, researchers can upload most files (usually Word documents or pdfs) to HawkIRB as attachments. However, due to the 10 MB size limit for files attached to the HawkIRB application, researchers need alternative ways to give the UI IRB access to large media files.
Researchers may provide electronic access to large recordings by:
Posting on YouTube or some other available platform and including a hyperlink in the application. If the platform requires using a login to view the media file, provide the details for viewing in the IRB application
Embedding a link in a Qualtrics or REDCap survey instrument and including the link in the application
Saving the file in OneDrive and sharing it with a Human Subjects Office (HSO) staff member who is involved in the review
If the third option is selected, the HSO staff member will save the file into a SharePoint site available to HSO staff and IRB chairs so the IRB members involved in the review process can view it.
Questions about this topic? Email the HSO Education and Outreach team or come to Office Hours to speak directly to a member of the HSO team.
When a principal investigator is planning to leave the UI, they need to inform the IRB and make changes to their active projects in HawkIRB and, if appropriate, ClinicalTrials.gov.
As soon as the PI knows their departure date, they should
A. Submit the appropriate form to the IRB. Either:
a Modification Form to change the PI (if the study is not complete and research activity will continue at the UI under a new PI), or
a Project Close Form (if the research will no longer be conducted at the UI).
B. Update the study’s associated ClinicalTrials.gov record to reflect the PI change if study activities will continue at the UI. If the study is complete and results are not required, the record should be closed. Submit the results if applicable regulations require them. If the study is ongoing and will continue at the PI’s new institution, notify the PRS administrator to initiate a transfer of the active record.
Planning for HawkIRB Access
Once a PI has left the UI, they will no longer have access to the HawkIRB system and are considered “deactivated” when their UI appointment ends. This status depends on their UI employment (HR) or enrollment (registrar) records. If the PI has HawkIRB delegates, the delegates can complete project closure forms and modification forms, but only for the projects to which they have delegate access rights. (See the article in the January IRB Connection Newsletter for more information on delegates.)
Submit a Modification Form:
The PI or delegate should submit a Modification Form if:
The study is not complete, and a new PI will be named on the application, and/or
To request IRB approval if the PI will take research data/samples to a new institution or plans to have continued access after departure.
To create a Modification form, log into your HawkIRB inbox. Under ‘Projects,’ click on the IRB number of the study you would like to modify.
Under ‘Create Form’ click on the Modification/Update Form
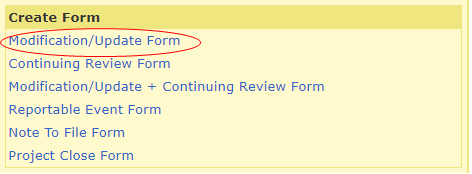
After starting the modification, you will be routed to the Frequent Mods portion of the Modification Index. If you will be sharing data, please see the November 2023 IRB Connection article ‘Best Practices for Research Collaboration and Data Sharing’.
To change the PI, select the option ‘Change the Principal Investigator.’ Enter the information of the new PI and select the ‘Set Principal Investigator’ button and then save the form.
To share or take data from the UI, modify section X.5 and 6. Please see the article November 2023 IRB Connection article ‘Herky Hint: What to do with all that Data? Data Sharing and Security Guidelines’.
To Submit a Project Close Form:
Departing researchers should not close projects unless they have completed all the following:
Protocol-indicated research activities, including interaction with subjects and collection of data or specimens
Collection of data about subjects even when no subject contact is necessary
‘Cleaning’ of data
Analysis of identified or coded data for research purposes or during the publication process
Any other research use of data that involves access to identified or coded data or specimens collected during the conduct of research
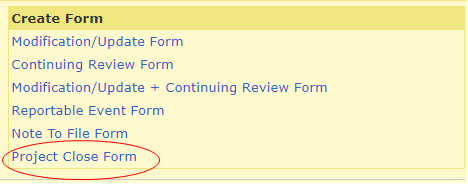
To create the form, log into your HawkIRB inbox. Under ‘Projects,’ click on the IRB number of the study you would like to close.
Under ‘Create Form,’ generate a Project Close Form.
The project will close as soon as the form is submitted, and the research team may no longer collect data about any of the subjects in the study, contact the subjects for research purposes, or work with identified data/samples.
If you will be collecting follow-up data the project should remain open, even if new subjects are not being enrolled. See the UI Guide Sec. II, Part 22. Questions about this topic? You can email the HSO Education and Outreach team or come to Office Hours to speak directly to a member of the HSO team.
Have an idea for an IRB Connection newsletter article? Let us know!