
What is an IND?
An IND is an application submitted to the Food and Drug Administration (FDA) whereby a drug sponsor requests authorization from the FDA that will allow the interstate transport of investigational agents for administration to humans. The sponsor may be an individual, pharmaceutical company, government agency, academic institution or private organization. For additional information on the content and format of IND application, see 21 CFR 312.23.
What is a Sponsor?
A sponsor is an individual or other entity that takes responsibility for and initiates a clinical investigation. The sponsor does not actually conduct the investigation.
What is an Investigator?
An investigator is an individual who actually conducts a clinical investigation. S/he is responsible for the administration and/or dispensing of the test article. If a team of individuals conducts the research, the investigator is responsible for the conduct of the team.
What is a Sponsor-Investigator?
A sponsor-investigator (SI) is an individual who initiates (i.e., obtains an IND) and conducts an investigation and under whose immediate direction an investigational drug is administered, dispensed, or used.
When an investigator holds an IND for the product being tested in a particular research study, s/he must also assume all the responsibilities of the sponsor, and is called a “sponsor-investigator.” Therefore, the regulatory requirements of a sponsor- investigator include those applicable to an investigator and a sponsor. [21 CFR 312 Subpart D].
What is a Clinical Hold?
A clinical hold is an order issued by the FDA to the sponsor to delay a proposed clinical investigation or to suspend an ongoing investigation. The clinical hold may apply to one or more of the investigations covered by an IND. A complete clinical hold is a delay or suspension of all clinical work under an IND. A partial clinical hold is a delay or suspension of only a part of the clinical work under an IND.
What is a FDA Form 1571?
The FDA Form 1571 or ‘1571’ is the IND application cover page and it must accompany the initial IND submission and any amendments, IND safety reports, annual reports or general correspondence the sponsor submits to the FDA about the IND. The 1571 is a contractual agreement between the sponsor and the FDA. By signing the 1571, the SI agrees to the following:
- S/he will not begin the clinical investigations until 30 days after the FDA’s receipt of the IND, unless the sponsor receives earlier notification from the FDA
- S/he will not begin or continue the investigations covered by the IND if the FDA has placed the investigations on clinical hold
- S/he will conduct the investigation in accordance with all other applicable regulatory requirements
- An IRB that complies with 21 CFR 56 will be responsible for initial and continuing review and approval of each of the studies in the proposed clinical investigation under the IND
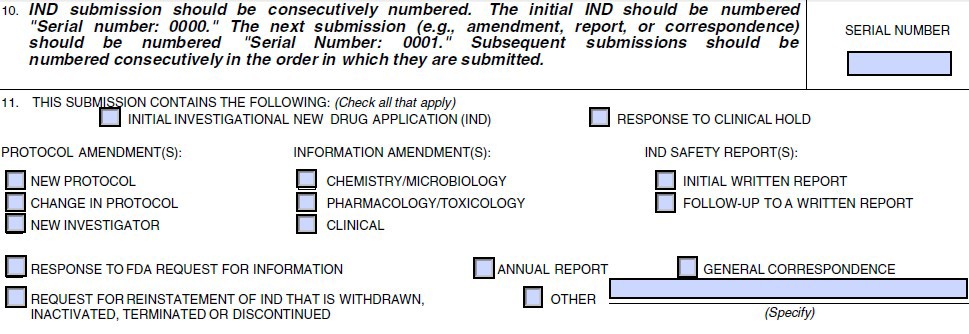
Submission to FDA: IND submissions should be consecutively numbered. In Section 10 of the 1571, the initial IND submission should be numbered ‘0000’; and subsequent submissions should be numbered consecutively in the order they are submitted (0001, 0002). For the initial IND submission, only the ‘Initial Investigational New Drug Application (IND)’ box should be checked in Section 11 of the 1571. Because subsequent submissions may contain more than one type of information, all boxes that correspond to the new information included in the submission should be checked. The FDA website states that the initial IND submission and each subsequent submission to the IND must be submitted to the FDA in triplicate (the original and two photocopies are acceptable); however, the requirements of individual reviewing divisions may vary. The SI should confirm the requirement with the FDA contact person assigned to the IND application review. (See Section III of this guidance).
What is ClinicalTrials.gov?
ClinicalTrials.gov is a clinical trial registry and results data bank operated by the National Institutes of Health (NIH). Section 801 of the FDA Amendments Act (Food and Drug Administration FDAAA 801) requires the registration and submission of the results of applicable clinical trials – which include those involving FDA INDs. The federal registration deadline is no later than 21 days after enrollment of the first participant. If there is a plan to publish the data, the International Committee of Medical Journal Editors requires registration prior to the enrollment of the first subject. After submission to CT.gov, the review process can take up to 30 days. Once accepted, the record including the CT.gov registration number or the National Clinical Trial (NCT number) will be available on their website within 2-5 business days. Currently at the UI, investigator-initiated studies must document the PI as the "responsible party". If the UI is the IRB of record, you will need to provide the NCT number in HawkIRB Section VII.B.1.b. Contact the PRS Administrator for assistance obtaining a username with the CT.gov Protocol Registration and Results System.
What is a FDA Form 3674?
The FDA Form 3674 is a ‘Certification of Compliance' to assist with meeting the requirements of Clinical Trials.gov Data Bank outlined in 42 U.S.C. § 282(j)(5)(B), section 402(j)(5)(B) of the Public Health Service (PHS) Act. This requirement went into effect on December 26, 2007. The form provides the FDA with the information required of applicants who submit certain human drug, biological product, and device applications, including Investigational New Drug Applications (IND) and new clinical protocols submitted as an amendment to an existing IND.
Submission to the IRB: The IRB expects the SI to scan and attach to the IRB application the entire contents of the submission that was sent to the FDA. This includes the following documents: initial completed/ signed 1571, protocol and the investigator’s brochure. This must be clearly labeled and included in the ‘Sponsor Documentation’ category on the Attachments Page. If these are submitted as one document, the IRB may request that they are separated into individual documents and attached in the appropriate category (protocol, sponsor documentation). In addition, either documentation showing the date the FDA received the application or FDA approval to proceed is required in the Sponsor Documentation category.
At the time of subsequent IND submissions to the FDA, the SI should also notify the IRB of the submission via a modification to the IRB Application. The corresponding 1571 should be uploaded ‘’on top’ of the previous 1571 version(s) on the IRB Attachments Page, using the ‘Edit Electronic Attachment’ instructions. The IRB modification should include an attachment documenting that the amendment was sent to the FDA and the date it was sent.
What is a FDA Form 1572?
The Form FDA 1572 or ‘1572’ is also called the ‘Statement of Investigator’. It is a legally binding contract between the investigator and the FDA, whereby the investigator agrees to provide specific information to the sponsor and assures that s/he will comply with FDA regulations related to the conduct of a clinical investigation of an investigational drug.
By signing this form, the investigator assumes full responsibility for the study, attests that s/he has read the Investigator’s Brochure and agrees to conduct the study according to the protocol and FDA regulations.
Each investigator that conducts the clinical trial and under whose supervision the study drug is administered must complete a 1572 prior to participating in an IND study. Additionally, s/he must update any changes to the information during the course of the study.
The PI is not required to attach the 1572 to the IRB Application.


