The Human Subjects Office (HSO), in collaboration with the Iowa Clinical Trials Management System (I-CTMS) Admin Team and the Institute for Clinical and Translational Science (ICTS) Regulatory Core have been working hard to develop a process to enhance timely release of modifications of any updated informed consent documents (this includes all informed consent-related document categories attached in HawkIRB) approved by an external IRB.
Effective immediately, the submission process for modifications approved by an external IRB has changed in HawkIRB. Studies utilizing the I-CTMS/Oncore (yes to question V.27 or V.22) will now automatically push over external IRB-approved informed consent documents for immediate use in the I-CTMS. This push will occur upon submission to the HSO for review of local content.
When submitting a modification,
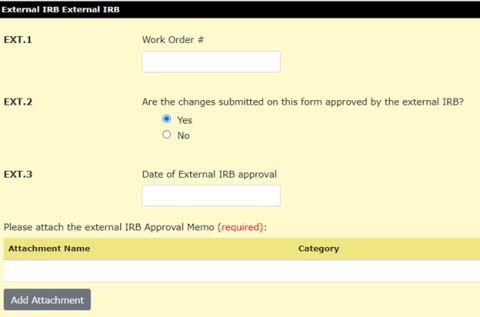
- If using WCG (WIRB-Copernicus Group) as the IRB of record, add the work order# in Ext.1
- Indicate if the external IRB has approved the contents of the modification in Ext.2
- Indicate the date of IRB approval in Ext.3
- Attach a copy of the external IRB approval memo
- Complete the modification as you would normally.
- There are now two attachment sections for each informed consent document attachment category (e.g. primary informed consent document, consent summary, assent, HIPAA Authorization, and other informed consent documents) on all external IRB applications.
- Attach the final IRB-approved informed consent document in the section designated as “clean” copy in the appropriate informed consent document attachment category.
- Attach the track changes version of the informed consent document in the “track changes” section in the appropriate informed consent document attachment category.
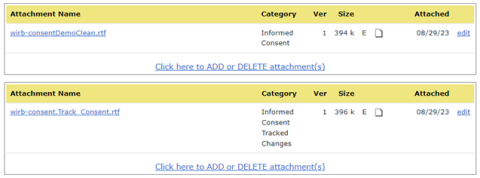
Utilizing two informed consent categories allows the HSO and the HRPP (Human Research Protection Program) staff to easily identify what changes were made in informed consent documents approved by an external IRB. HawkIRB will also automatically push any informed consent documents into the I-CTMS for immediate use at the point the modification is submitted to the HSO for review. If changes are necessary to attachments or contents in the modification, the HSO and/or the HRPP committee will notify the research team as we have in the past. If changes are necessary to address local context issues, the study team will be required to submit a new modification to the IRB of record to address the items.
HawkIRB will also continue to push updated protocols and Investigator Brochures to the I-CTMS/Oncore for all studies indicating "yes" to either V.22 or V.27, upon HSO release of the modification.
Please note: You are not required to submit a modification to the external IRB for approval prior to submitting a modification for local context review. In the event a pre-local context review is required before external IRB review, simply answer “no” to Ext.2 indicating there is not IRB approval and the HSO External IRB team will work with you to complete the local context review prior to external IRB submission.
Contact the Human Subjects Office at (319) 335-6564 or email uirb-external@uiowa.edu if you have questions or need assistance.


