Index
Four-part HawkIRB Training Series Recordings Now in ICON
The Student PI Training Requirement is Now in Effect
HawkIRB Enhancements
Reminder: Pre-Grant Submission Survey for Single IRB Model
Hot Topics & IRB/HRPP Updates
Social, Behavioral and Education Researchers: There is an IRB tool just for you!
Locating Support and Training Materials for CT.gov
IRB Advisor Newsletter, July 2021
In the News
Four-part HawkIRB Training Series Recordings Now in ICON
The Human Subjects Office (HSO) is pleased to announce that full recordings of all four parts of the HawkIRB Training series are now available through the IRB ICON Course for Researchers. Researchers’ schedules do not always allow for attendance at a live session. These recordings provide the UI research community with 24/7 access to the most up-to-date information about how to complete HawkIRB forms.
Note: To access the Part 2 recording, you must complete a quiz after viewing the Part 1 recording. There are no prerequisites to access the recordings for Parts 3 and 4.
The Human Subjects Office will continue to offer live, hybrid training sessions for researchers who prefer this style of learning. A full schedule of the HawkIRB training sessions can be found on the Education and Training page of the HSO website. Contact the Education and Outreach Team if you have any questions.
The Student PI Training Requirement is Now in Effect
By Kelly O'Berry, BS, CIP
Effective August 23, 2021, before a student Principal Investigator (PI) submits a New Project form in the HawkIRB system, they must attend Parts 1 and 2 of the HawkIRB training series OR view recordings of these sessions and complete quizzes in the IRB ICON Course for Researchers. This training requirement is intended to improve the quality of HawkIRB submissions and the efficiency of the IRB review process. This new policy was announced in the June 2021 IRB Connection Newsletter, in the July 1 News from the Office of the Vice President for Research newsletter email, and via other UI campus communication channels.
HawkIRB System Changes
In Section II.3 of the HawkIRB application, when the PI is a graduate student, undergraduate student, or other, there is a message with a hyperlink to the policy announcement and a statement about whether the training requirement was completed.
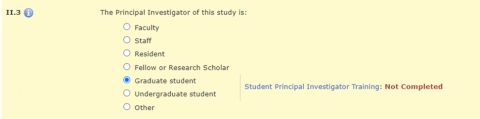
OR

There is a hard stop on moving beyond Section II.3 until the student PI completes this training requirement.
Training Options
Check the Education & Training page of the Human Subjects Office website for the current schedule of HawkIRB trainings and the portal to the IRB ICON Course for Researchers.
Certified Investigator Database
Once a student PI attends the live (in-person or via Zoom) trainings or completes the quizzes after viewing the recordings, it will take up to two business days to be added to the Certified Investigator Database. Once added to the Database, the HawkIRB system will recognize that training is complete and allow the student PI to continue filling out the draft New Project form.
If a student PI previously attended the live (in-person or via Zoom) HawkIRB trainings but does not appear in the Certified Investigator Database, send the dates of attendance to irb-outreach@uiowa.edu. We will verify attendance at the HawkIRB New Project trainings (Parts 1 and 2) and update the database.
Please direct all questions and comments about this new training requirement to irb@uiowa.edu.
HawkIRB Enhancements
In response to requests from the research community, the Human Subjects Office (HSO) has added two enhancements to HawkIRB. Those changes, effective on Wednesday, September 15, 2021, are:
- Researchers can now indicate if a study is closed to (or has permanently stopped) enrollment of new subjects via the modification form rather than waiting until the continuing review is due. (Question XIII.2)
- Researchers can select the Consent Summary (or Key Information Sheet) as a tool to obtain informed consent. (Question VII.D.15)
Applications submitted through IRB-01 will electronically share this information with the UI HCIS (Healthcare Information Systems) and the HCCC (Holden Comprehensive Cancer Center) IT teams to ensure accurate information is captured in the EPIC Research module, the UI Health Care Clinical and Research Trials Website, and Oncore.
Additional enhancements to HawkIRB that have already been made or will be rolling out soon are summarized in the Hot Topics & IRB/HRPP Updates article in this issue of the IRB Connection.
If you have ideas for additional HawkIRB enhancements, please submit them to irb@uiowa.edu for consideration by the HSO.
Reminder: Pre-Grant Submission Survey for Single IRB Model
Federal agencies require the use of a single IRB (sIRB) for federally funded research conducted at multiple sites. This means one IRB oversees research conducted at some or all of the study sites. The budget must include the fees for this type of IRB review and this type of research may require some additional approvals and agreements. Researchers can access information about UI IRB fees for budget planning.
If you are planning to submit a grant proposal to the National Institutes of Health (NIH) or any other federal agency that requires the sIRB model, complete the Pre-Grant Submission Survey as soon as you become aware of the award notice. Plan ahead and complete this survey well in advance, especially if the UI IRB is either serving as a lead IRB or relying on an external IRB as an expectation of the grant!
If you have questions or need any assistance, contact the External IRB Team at uirb-external@uiowa.edu .
Hot Topics & IRB/HRPP Updates
By Kelly O'Berry, BS, CIP
In July 2021, the Human Subjects Office (HSO) initiated a lecture series to share information about hot topics and updates from the Institutional Review Board (IRB) and the Human Research Protection Program (HRPP). These lectures will occur three times a year (July, October and March) to address common questions, new initiatives and HawkIRB enhancements for the UI research community. This article provides a recap of the information covered in the July 2021 lecture. A recording is available in the Additional Topics section of the IRB ICON Course for Researchers.
HSO Staffing
The HSO and IRB teams are pleased to welcome six new staff members. They are:
- Carly Okken, Project Specialist
- Fozia Ghafoor, Clinical Trials PRS Administrator
- Lance Hanson, Application Analyst
- Laura Shinkunas, IRB-01 Chair Designee
- Mayzie Tucker, Commercial IRB Coordinator
- Svetha Swaminathan, Senior Application Analyst (who moved from the IRB Coordinator position she held for over a year and a half)
There are additional open positions in the HSO, so keep an eye out for job postings if you or someone you know may be interested and/or qualified to apply.
Single IRB (sIRB) Pre-Grant Submission Survey
The UI IRB uses a survey to collect information about research using the single IRB (sIRB) model, whether the UI is the lead site and lead IRB or if we are relying on another IRB. Researchers should submit the sIRB Pre-Grant Submission Survey when they first become aware of the award notice for a federal funding source that requires use of the single IRB model. This is required for all federally funded, multi-site research.
There is a link to this survey on the Central and External IRBs (Single IRB of Record) page of the Human Subjects Office website. There is also guidance on that page for using a central or commercial IRB.
Student PI Training Requirement
Effective August 23, 2021, all student Principal Investigators (PIs) are required to complete HawkIRB New Project training Parts 1 and 2. This educational requirement is intended to help student PIs learn how to submit more complete applications that go through the IRB review process more smoothly.
This new training requirement was also announced in the June IRB Connection Newsletter. This issue of the IRB Connection Newsletter includes additional information about the HawkIRB programming for this training requirement.
Applications Not Ready for Review
In the July IRB Connection Newsletter, the IRB announced a new policy for returning applications that are not ready for review. This policy took effect at the beginning of the semester on August 23, 2020. The HSO/IRB will now return HawkIRB applications, without review, for the following reasons:
- Submission does not meet federal requirements for IRB approval (45 CFR 46.111 or 21 CFR 56.111)
- Incomplete description of study
- Partial answers (especially to multi-part questions)
- Multiple inconsistencies – within the application or between the application and attached documents
- Problems with documents attached to the application
When HawkIRB applications are received that are not ready for review, the HSO staff reviewer will route the form back to the PI through workflow with a referral to educational resources for preparing a more thorough, detailed application.
New Research Integrity Officer
Mike Andrews, BS, MBA, oversees the research ethics program, which includes the Responsible Conduct of Research training program. He coordinates and oversees the UI response to regulations and requirements for foreign influence in research. This includes investigations of:
- Allegations of research misconduct (fabrication, falsification, plagiarism)
- Allegations of foreign influence in research, including failure to report conflicts of interest and commitment
- Other allegations of unethical behavior
HawkIRB Enhancements
Since HawkIRB is a homegrown eResearch application system, improvements and enhancements are made as programming time allows. There are several HawkIRB updates that have already been rolled out or are coming soon:
- Delegate Permission System – HawkIRB Delegates now see the PI names in alphabetical order. Delegates also see PIs who are “deactivated” (no longer at the UI). The Delegate is now able to submit Modification or Project Close forms and respond to workflow on behalf of a deactivated PI to ensure appropriate research oversight. This update is live.
- Individual Investigator Agreement (IIA) – We added an intake sheet for non-UI research team members. This update is live.
- Holden Comprehensive Cancer Center (HCCC) Protocol Review and Monitoring Committee (PRMC) – We added a link to the PRMC form that was formerly on paper. This update is live.
- Human Subjects Research Determination (HSRD) forms submitted by a student PI – HawkIRB sends an email notice to the Faculty Advisor when a student PI submits an HSRD form. This update is live.
- Section II.6 (Key Personnel) – Clarified eCOI disclosure status for Key Personnel. This update is live.
- Section VII.D.15 (Materials used to obtain/document informed consent) – We will be adding the Consent Summary of Key Information as an option in the list of materials. This will make the response to Section VII.D.15 be consistent with the documents attached in the Consent/Assent Documents attachment category. This update is effective on Wednesday, September 15th, 2021.
- Closed to Accrual Status Changes – Currently, researchers can only indicate when a study is closed to enrollment on a Continuing Review form. When this update is rolled out, researchers will be able to indicate a change in accrual status on a Modification form. HawkIRB will still feed changes in accrual status to UIHC services such as Epic Research Module and the UIHC Clinical Research and Trials website. This update is effective on Wednesday, September 15th, 2021.
- Exempt Application Forms – The largest HawkIRB enhancement initiative since 2008, is a complete overhaul of the HawkIRB form for studies that qualify for Exempt Status. The forms will be shorter and will include all exceptions to the regulations that are allowed for this type of research. The biggest changes will be in Section IV.1 where researchers select the type of IRB review. HSO staff and IRB Chairs are currently reviewing the programming changes. We would appreciate volunteers from the UI research community to assist with pilot testing in September-October 2021. To volunteer, contact Michele Countryman (michele-countryman@uiowa.edu). Depending on the outcome of the pilot testing, the anticipated launch date is November-December 2021. This update is coming toward the end of the year.
- HawkIRB Application Redesign – The IRB Taskforce recommended that we explore ways to simplify the HawkIRB application. We have been working with partners in Holden Comprehensive Cancer Center and the Institute for Clinical and Translational Science (ICTS) to streamline the application and clarify IRB or UI/UIHC expectations. This update is anticipated in 2022.
UIHC Data Governance Committee
UI Health Care has a Data Governance Committee (DGC) that establishes policies and oversees activities related to the use and sharing of data from Epic and other UIHC sources. The charter has been defined and the committee is currently working on establishing a formal policy for researchers to share data outside UIHC. Stay tuned for future updates!
We welcome your suggestions for topics to include in future Hot Topics and IRB/HRPP Updates presentations. Please send your ideas to irb@uiowa.edu.
Social, Behavioral and Education Researchers: There is an IRB tool just for you!
By Lisa Polakowski Schumacher, PhD
Research that focuses on lived experience, social and behavioral interventions, and education outcomes or practices requires that different information be included in the informed consent document than for biomedical research. Additionally, there could be some language in the Informed Consent Document (ICD) template that does not apply to a social science study. The Informed Consent Document Checklist for Social, Behavioral, & Educational Research was developed and designed to pare down the original Informed Consent Document Checklist to include only relevant information for Social-Behavioral and Education Research (SBER).
During the Institutional Review Board (IRB) review process, staff reviewers look for consistent information between each section of the HawkIRB application AND the required attachments. There are two steps in the review of a HawkIRB application that must occur before the application can move to final review and approval by the IRB or an IRB Chair:
- Administrative review (or pre-screening)
- Human Subjects Office (HSO) review staff initially look at a study to ensure all required documents are attached (i.e. Assurance Document, ICD, recruitment materials).
- If there are missing documents, HSO review staff will send the HawkIRB application back to the Principal Investigator (PI) through workflow to add the documents.
- HSO review staff also perform a cursory look at the consistency in each section of the HawkIRB application
- If there are glaring inconsistencies, HSO review staff will send the study back to the PI through workflow to request more information about the inconsistencies.
- Senior staff review
- Senior HSO review staff look carefully at each section of the HawkIRB application to ensure answers are consistent with:
- federal regulation requirements.
- institutional policy requirements.
- all corresponding sections of the HawkIRB application.
- all required documents.
- If there are any inconsistencies, the HSO review staff will route the study back to the PI through workflow to fix the inconsistencies.
- Senior HSO review staff look carefully at each section of the HawkIRB application to ensure answers are consistent with:
HSO review staff do not move the HawkIRB application to the final step in the approval process (i.e. IRB or IRB Chair) until the HawkIRB application meets federal regulations, institutional policies, and an acceptable level of consistency.
Thus, each time HSO review staff send workflow questions back to the PI, IRB approval of the study is delayed. The Informed Consent Document Checklist for Social, Behavioral, & Educational Research facilitates consistency across the entire HawkIRB application and between the application and the attachments; it may help to decrease the time it takes for studies to get approved by IRB.
Share this resource, the Informed Consent Document Checklist for Social, Behavioral, & Educational Research, with your research peers and graduate students to help reduce inconsistencies across the HawkIRB application!
Locating Support and Training Materials for CT.gov
Due to a technical error, this article will appear in the next issue of the IRB Connection Newsletter. We apologize for any inconvenience.
IRB Advisor Newsletter, July 2021
By Kasey Lockett, BA
IRB Advisor (a publication of Relias) is a monthly newsletter with articles about issues facing IRBs, Human Research Protection Programs (HRPPs) and researchers. Current and past issues of IRB Advisor are posted in the “IRB ICON Course for Researchers” which is accessible to anyone with an active UI HawkID. The portal to this ICON Course is on the Education and Training page of the Human Subjects Office website.
This month we highlight an article from the July 2021 issue about public support of COVID-19 human challenge trials, in which participants would be exposed to the virus after they were given the vaccine in order to test its efficacy.
Study Results Show Public Support for Alternative Vaccine Design
In May of 2020, researchers began a study to see what people thought about accelerated clinical trial vaccine designs. Primarily, they were interested to see what the public’s opinion was on accelerated designs and if they would be viewed as ethical. This study is noteworthy because one function of IRBs is to determine societal attitudes to help determine what is ethical, according to Yale University professor, Joshua Kalla. Additionally, it follows along with a document on accessibility of COVID-19 studies, put out by the World Health Organization, which stated that challenge research programs should be informed by consultation and engagement with the public.
The study was carried out through a large online survey across multiple English-speaking countries. The participants were presented with two fictional trial designs: a status quo approach and a human challenge design. Details included the number of volunteers proposed, a vaccine group and a placebo group. Participants in the survey were made aware that the placebo group in these fictional studies would be exposed to COVID-19, but that they would all be young, so serious complications or death would be unlikely. Results showed that most study participants preferred an accelerated design and found it to be ethical. Across countries, among older people, people of color, and people who lived in places with higher COVID-19 cases – 75% thought the human challenge trial was ethical and 6% thought it was not ethical.
The findings of the study suggest that IRBs should not assume that accelerated designs of clinical trials are ethically unacceptable to the public. A further implication is that IRBs may want to expand their thinking beyond the single community member serving on the board. Using tools like surveys to get a more extensive idea of what a community person thinks about a study can help eliminate biases that may occur when limiting an IRB to the view of a single member.
Additional Articles in this Issue:
- IRB Project Cuts Protocol Modification Time in Half
- Q&A: Data Safety Monitoring Board Experts Explain Role in Clinical Research
- IRB Approaches Research Participant Complains Individually
- Study Author Gives Recommendations to Improve Research Dispute Process
- When Complaints Are Not Resolved
In the News
- FDA threatens second company with fines over missing clinical trial results, TranspariMED
- FDA Hits Second Firm Over Alleged ClinicalTrials.gov Reporting Noncompliance, The Association of Clinical Research Professionals (ACRP)
- Clinical Investigator Warned for ClinicalTrials.gov Noncompliance, CenterWatch
- Wildfire smoke exposure during pregnancy increases preterm birth risk, EurekAlert!
- Faulty memories of our past whereabouts: The fallacy of an airtight alibi, ScienceDaily
- Poop: The Newest Disease Detection Tool for COVID-19 and Beyond, US News & World Report



