Index
National Cancer Institute (NCI) funded research occurring at the Iowa City VAHCS
Announcing New Policies Regarding IBC Review
Other Committee Review Tool
National Cancer Institute (NCI) funded research occurring at the Iowa City VAHCS
By Michele Countryman
VA Health Care System (VAHCS) researchers who have funding from the National Cancer Institute (NCI) can now submit projects to the NCI Central IRB (CIRB). The Iowa City VAHCS has established an IRB reliance relationship with the NCI CIRB to conduct these reviews. Similar to the University of Iowa (UI) NCI CIRB submission process, there will be a VA institutional review of these submissions to ensure all VA Human Research Protection Program (HRPP) review and approvals are in place. This VA HRPP review occurs before the NCI CIRB review and approval of projects. The University of Iowa Investigator's Guide and the VA Investigator's Guide have been updated to reflect this change.
HawkIRB Updates
We updated Question I.1 of the HawkIRB to allow for the selection of either the UI NCI CIRB or VA NCI CIRB. NCI funded research occurring at both the UI and VA will require two separate NCI CIRB submissions to ensure appropriate UI/VA items are addressed.
Prior to submission to the NCI CIRB, the VA Research Office will review the HawkIRB submission to ensure compliance with all VA institutional requirements, such as Pharmacy and Therapeutics Committee, Medical Radiation Protection Committee, HIPAA Privacy Protections, and others.
For questions about the VA NCI CIRB submission process, please contact the VA Research Office at 338-0581 x7678.
Announcing New Policies Regarding IBC Review
By Kelly O'Berry
In an effort to streamline the Human Research Protection Program (HRPP) review process for studies requiring review by other committees/entities, the IRB recently revised the policies regarding the Institutional Biosafety Committee (IBC) review of studies that involve the use of recombinant DNA. The two revised policies have to do with the timing of IBC review and limits on IRB oversight of these projects.
Timing of IBC Review
The IRB used to require IBC approval before a New Project form could be scheduled to an IRB meeting. Effective immediately, IBC review can be concurrent with the IRB review process. The HSO will hold the release of the project to begin the research until documentation of IBC approval is in place.
 IRB Oversight for Studies with Recombinant DNA
IRB Oversight for Studies with Recombinant DNA

HRPP policy used to require submission to the UI IRB for all studies that involve recombinant DNA. Effective immediately, research subject to IBC review can submit to an external or commercial IRB. IBC review can be concurrent with the external or commercial IRB review process. The HSO will hold the release of the project to begin the research until documentation of IBC approval is in place. The has been updated to reflect this change.
Additional Information
Please see the other article in this November 2019 Regulatory IRB Connection Newsletter that outlines some tools and resources for researchers regarding the other committees and entities in the Human Research Protection Program (HRPP) that also review and approve certain kinds of research projects in conjunction with the IRB review process.
Other Committee Review Tool
By Kelly O'Berry
Depending on the study design and procedures, there are other committees and entities that may need to review and approve a human subjects research project in addition to the IRB. Many of them use the HawkIRB application to conduct at least part of their review. The Human Subjects Office (HSO) has a web page that outlines the role of these other committees and entities. This article provides an orientation to the other committee review and some tools that are available on the HSO website.
The Other Research Review page of the HSO website includes a list and process flowchart of the other HRPP committees that have a role in reviewing human subjects research in conjunction with the HRPP/IRB review process. The Other Committee Tool posted on this page is intended to help researchers identify when Human Research Protection Program (HRPP) approvals are required. It is a three-page document that contains:
- a flow chart of the relationship between the HRPP committees,
- a graphic to help identify when other HRPP committee approval is required, and
- a table with the regulatory mandate for each of these other HRPP committees/entities
Flow Chart – Relationship Between Committees
The first page of the Other Committee Review Tool is an organizational chart that shows how the various units relate to one another. Some of the committees/entities operate under the Office of the Vice President for Research (OVPR) and others are overseen by UI Health Care (UIHC) or Holden Comprehensive Cancer Center (HCCC). The dark lines represent oversight responsibility between the individuals and offices in the white boxes and some of the committees (i.e., the Environmental Health & Safety Office oversees the Institutional Biosafety, Stem Cell and Medical Radiation Protection committees).
The dotted lines indicate the interrelationship between the committees and entities in the yellow boxes and their connection to the Institutional Review Board (IRB).
Researchers can use this flow chart to identify the lines of reporting and oversight between all of these offices and committees.
Graphic – When Other Approval is Required
The second page of the Other Committee Review Tool was created at the request of a new researcher to provide guidance about when a committee needs to approve a project. This color coded graphic can be used in several ways. First, this page includes a set of two-color boxes for each committee or entity that might need to review and approve a project. Use the dark boxes to figure out if the study needs approval from that committee. For example, if the study will provide compensation to subjects, approval is required from Accounting Services. Use the “Learn more” in each box to go directly to a web page with information about that committee or entity.
The second way to use this graphic the color coding to know when committee approval is required in relation to IRB approval. The colors of the dark boxes indicate which approvals are required before IRB review and which ones are current, after or completely separate from IRB approval.
- When other committee approval is required before IRB review (green), Human Subjects Office (HSO) staff can begin the application review process, but they cannot schedule the application to an IRB meeting until the other approvals are in place. This ensures that the IRB reviews the final study design and procedures that comply with all UI requirements related to cash handling, conflicts of interest, research billing and research at the Holden Comprehensive Cancer Center.
- When other committee review is concurrent with IRB review (teal), the application can be reviewed at an IRB meeting but the IRB cannot approve the study until it is approved by the committee. These committees (Pharmacy & Therapeutics, Nursing Research, Medical Radiation Protection and ClinicalTrials.gov) could request additions or changes in the HawkIRB application that the IRB would need to review and approve.
- When the project has an investigational drug that requires dispensing by the UIHC Investigational Drug Service (IDS), recombinant DNA, or has external funding, the IRB can approve the project but holds the release until the committee approval or grant/contract is in place (dark purple). The timing of IDS review changed in 2018.
- There are two entities whose review is completely separate from the IRB review (lavender). The Department of Pathology Services and the Clinical Research Unit (CRU) have their own application forms and processes that are not integrated with the HawkIRB application.
- There is only one committee that reviews the application after IRB approval (blue), the Veterans Administration Research and Development (VA R&D) Committee. The IRB holds the application to release after VA R&D Committee approval.
Important Note: When a project will be overseen by an external IRB, the researcher must obtain all but IDS, DSP, and IBC approvals before submitting an application to the external IRB. This ensures compliance with all institutional policies and procedures that an external IRB would not oversee.
Table - Regulatory Mandates
Just like the IRB, each of the HRPP committees follow specific regulations and policies. The third page of the Other Committee Tool provides brief references to these regulatory mandates for each committee/entity.
Flowchart of the HRPP Review Process
This document includes a color-coded flowchart on the first page. The key to the color coding is at the top, below the heading. The rest of the pages include additional information about each of the committees/entities.
The HawkIRB Application
There are questions in Section V of the HawkIRB application about most of the other committees or entities. A “Yes” response to these questions triggers a notice to the committee about a new study that requires their review. In a HawkIRB Modification form, responses in Section XIV trigger a notices that a change in the study design requires their approval.
Please be aware that during the IRB review process for a New Project or Modification form, researchers must communicate directly with the committee or entity about any required actions. HawkIRB does not notify the committee or entity when a researcher routes a form back to the IRB during the review process. If a change was made at the request of an HRPP committee/entity, researchers must notify the committee that their requested changes have been made in the HawkIRB system.
In 2018, we made some changes to the HawkIRB system to make it easier for researchers to see what other committee reviews are still pending for a form. In the Pending Forms section of the PI’s inbox, it now shows the box the form is in and the other committee approvals that are required.
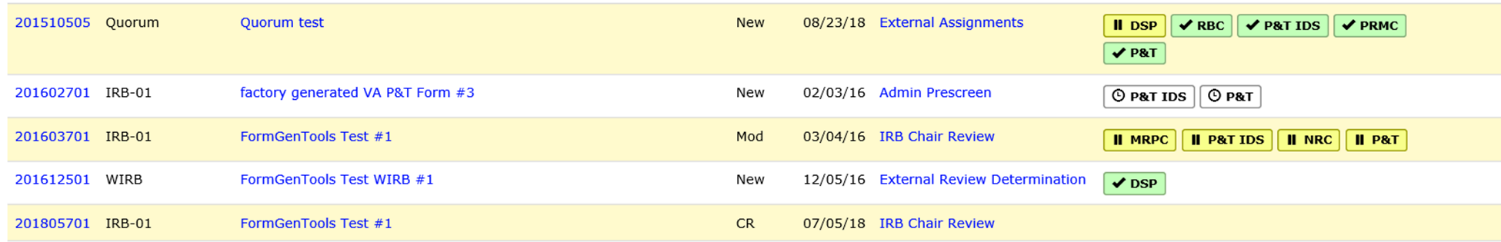
The boxes include the following color codes and symbols:
- White box / clock symbol – Means the form is still in the IRB pre-screening process and has not yet been sent to the committee or entity.
- Yellow box / pause symbol (II) – Means the notification was sent to the HRPP committee to complete their review and the form is pending HRPP Committee approval.
- Green box / check mark – Means the committee or entity approved the form.
Another way to obtain this information is at the bottom of the Project Summary page, in the History section. The PI or his/her delegates can click on the link in the Other Committee Review section to see which approvals are still pending.
If you have any questions about the application forms or review process, it is best to go directly to the committee or entity. More information is available on the Other Research Review page of the Human Subjects Office website.



