Index
OHRP Announces Release of Research With Children Video
Annual Disclosure of Outside Activities Due April 30
Herky Hints: How to Edit and Attach Revised Documents to HawkIRB
IRB Advisor Newsletter, February 2021
In the News
OHRP Announces Release of Research With Children Video
By Joanie Hoefer, BS, CIP
The Office for Human Research Protections (OHRP) provides several short informational videos for potential research volunteers that share basic information about human subjects research. These videos are designed to help potential research volunteers learn how research works, know what questions to ask before deciding to participate in research and know what things they should think about before participating. The newest video is for parents or guardians whose children are being asked to participate in a research study.
The ‘Research with Children: What Parents Need to Know’ video provides basic information about research with children, why it is important, and what parents should expect if they are considering enrolling their child in a study. This video can be found on OHRP’s informational videos page in the Videos on Protecting Human Research Volunteers section.
UI researchers are encouraged to share these video resources with their potential research subjects. For additional educational resources for researchers and subjects, please visit the OHRP Education & Outreach web page.
Annual Disclosure of Outside Activities Due April 30
By Martha Hedberg, MPA
All UI faculty and staff identified as key personnel that conduct human subjects research are required to complete an “Annual Disclosure of Outside Professional Activities and Interests” via the eCOI online disclosure portal by April 30, 2021. This article describes the disclosure requirements and highlights new questions that were added to the eCOI disclosure form in 2021.
The University of Iowa community is committed to the principle of free, open, and objective inquiry in the conduct of its teaching, research, and service missions. Further, the University of Iowa encourages its employees to engage in external activities that promote the University's mission, contribute to their professional fields, enhance their professional skills, and/or enhance the public good. To ensure that external activities are conducted in a manner consistent with institutional and public values, policies have been established to ensure that University employees avoid improper conflicts, and otherwise disclose activities for review and management.
Employees may monitor compliance with this annual disclosure requirement via the UI Compliance and Qualifications system accessible through UI Self-Service “My Compliances” tab. Employees who have not completed the requirement will receive reminder emails on April 1, April 25, and April 30. Supervisors and Compliance Administrators will be copied on the final two notifications. After April 30, employees may add new, review or edit previously submitted reports directly using https://ecoi.uiowa.edu.
Who Should Disclose
Under this policy, “key personnel” means anyone involved in the design, conduct and/or the reporting of the research. These roles are specified because they provide opportunities for the introduction of bias into the research. If you are unsure if this applies to you, check with the PI of any studies in which you are a study team member.
What to Disclose
Disclosures should be submitted to the University for any outside activities that are related to an employee’s role or area of expertise at the University. For more detail about what types of activities to disclose, see the Conflict of Interest in Research’s website.
New for 2021
Conflict of Interest in Employment
New this year will be a question asking whether the individual has an outside personal relationship with someone over whom they exercise supervisory authority. This addition will assist with identifying possible Conflict of Interest in Employment (Nepotism) cases that may require a management plan.
International Engagement
Also new this year are questions asking about international engagement. NIH Grants Policy Statement defines a Foreign Component or engagement as “any significant scientific element or segment of a project outside of the United States, either by the recipient or by a researcher employed by a foreign organization, whether or not grant funds are expended" and includes “collaborations with investigators at a foreign site anticipated to result in co-authorship; use of facilities or instrumentation at a foreign site; or receipt of financial support or resources from a foreign entity.”
International collaboration is often an important aspect of those activities. To ensure it is conducted in a manner consistent with institutional and public values, policies have been established to ensure that UI employees avoid improper conflicts by disclosing those relationships for review and management. Full and timely disclosure regarding research support and conflicts of interest is essential to ensure compliance with federal regulations and sponsor requirements.
Failure to disclose this information can have significant fiscal and regulatory consequences, impacting both the individual PI and the institution. This addition will assist with identifying possible risk to research integrity and security which may require conflict of interest management or adherence to other federal regulations.
Thank you for your contributions to ensuring that UI academic, health care, business, research, and teaching endeavors may be free of potential or actual conflicts of interest.
Institutional Policies
The Annual Disclosure fulfills the reporting requirements of the following UI policies:
- Policy on Conflicts of Interest in Research,
- Policy on Conflict of Interest in the Workplace,
- Policy on Conflicts of Commitment,
- Policy on Conflicts of Interest in Employment (Nepotism),
- Policy on Institutional Conflict of Interest in Human Subjects Research,
- UI Health Care Conflicts of Interest Policy, and
- Accreditation requirements for Continuing Medical Education.
Herky Hints: How to Edit and Attach Revised Documents to HawkIRB
By Joanie Hoefer, BS, CIP
When completing a new project application, researchers must sometimes make edits to original documents that they submitted to the IRB during the review of the application. Additionally, many modifications to a HawkIRB application also require changes to the existing IRB-approved documents. This article shows, step-by-step, how to correctly download a document for editing and upload the modified document so that its version history is retained throughout the lifecycle of the application.
Step 1: Download the Attachment from HawkIRB
It is important to download the document from the HawkIRB application instead of using a file stored on your computer.
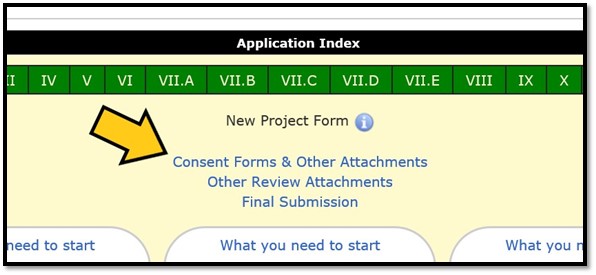
To get to the attachments page of a new project form, click on the ‘Consent Forms & Other Attachments’ link on the Application Index:
To get to the attachments page for a modification form, click on the ‘Attachment Changes’ tab of the Modification Index.
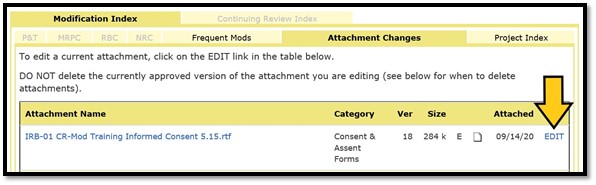
For either form, click on the ‘EDIT’ link to the right of the filename of the attachment you need to revise:
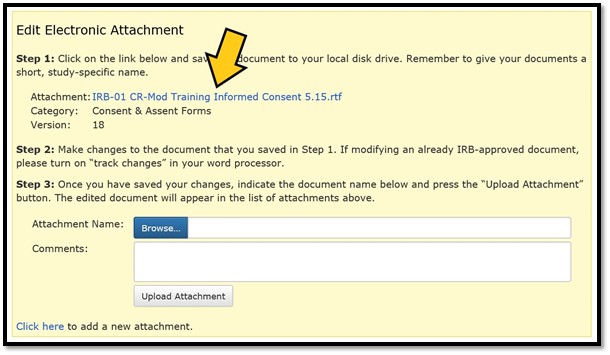
The edit box describes the three-step process. Click on the attachment link in Step 1 to open the document for editing.
Step 2: Edit the Document
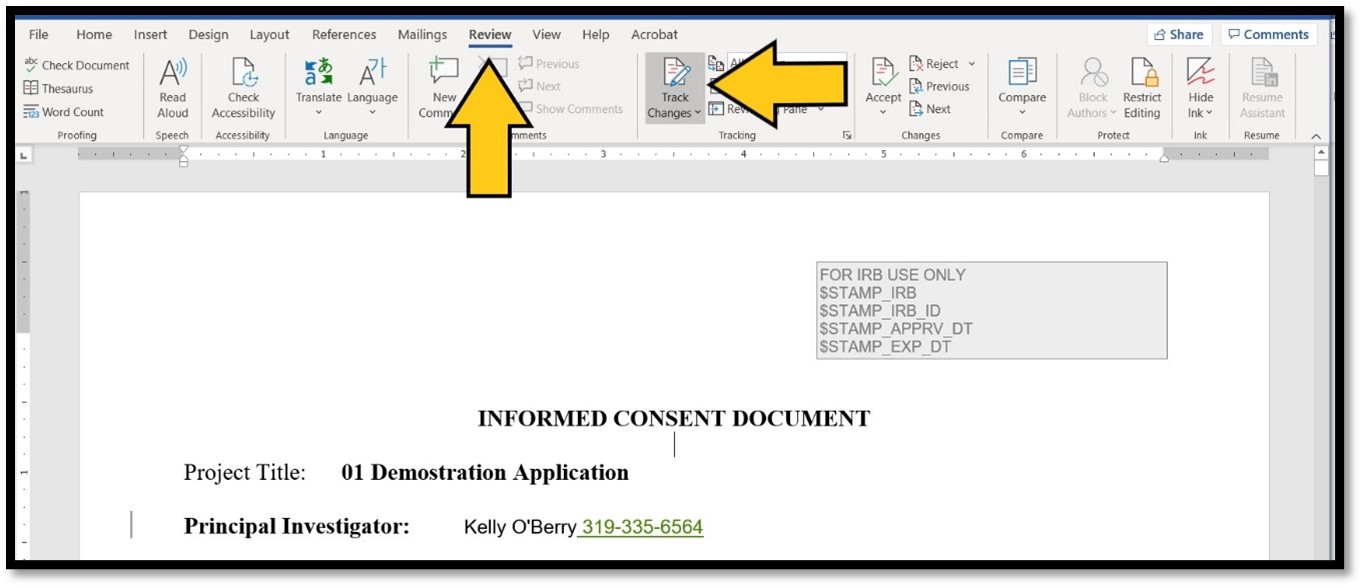
Save the document and make your edits. Any changes to an approved document that was created in a Word program should be made using the Track Changes function engaged. This allows the reviewer to easily identify the changes that have been made, resulting in a quicker review of the revised document. The Track Changes function is found on the Review tab in the Word document:
MAC Users! Macs do not always open .rtf documents in Microsoft Word by default. This causes the IRB stamp to be lost. This will result in an error message when you try to upload your revised document. Review the instructions on the HSO website to configure your Mac for .rtf documents.
For any documents that were created in a program that does not have the capability of tracking changes, clearly describe all the edits that were made to a document in workflow for a new project form and in Section XIII (Modifications and/or Other Comments) for a modification form.
Once you are done editing the document, save the changes. It is not necessary to use a new filename for the document as you will be stacking the newly revised document on top of the previous version in the next step. However, it may be helpful to use a naming convention to differentiate between the versions of the document saved on your computer so you upload the correct document in Step 3.
Step 3: Upload the Revised Document to HawkIRB
Attach the modified document as a new version of the document and not as a new attachment. You can see the version number of any attachment in the ‘Ver’ column on the Attachments page of a new project form or on the ‘Attachment Changes’ tab of a modification form:
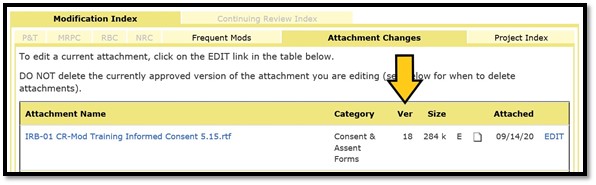
Return to the Edit Electronic Attachment box that opened in Step 1 and click on ‘Browse…’. If you navigated away from the window with the edit box while working on the document, simply click the ‘EDIT’ link to the right of the corresponding attachment filename to re-open the box.
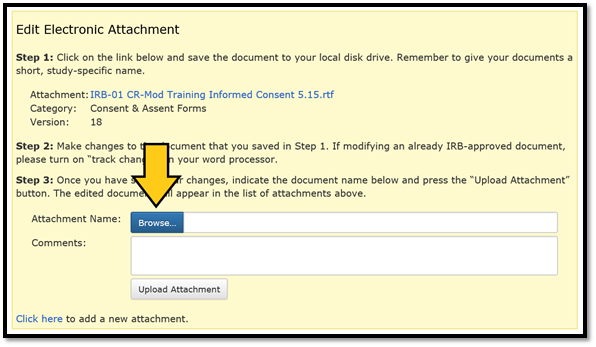
Search for your newly revised document and either double click on the filename or click on ‘Open’:
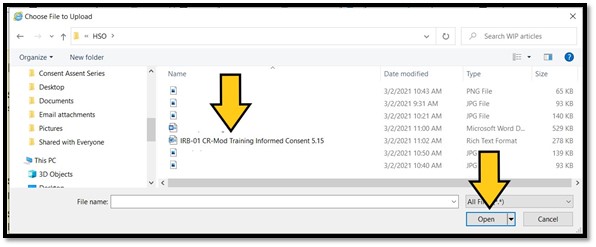
The filename should now appear in the text field next to the ‘Browse…’ button. Click on ‘Upload Attachment’ to complete the uploading process:
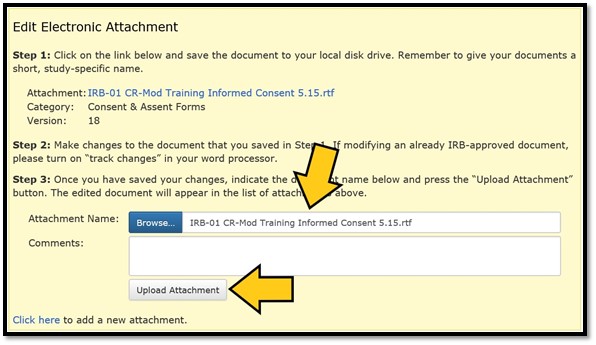
The version column will show that the version number has advanced by one. To see all previous versions of the attachment, click on the ‘+’ to the left of the filename:

DO NOT DELETE previous versions of any attachment unless you will no longer use the attachment in the study. A Human Subjects Office staff reviewer might ask you to delete an attachment. When study recruitment is complete, researchers should typically delete all recruitment materials and the Informed Consent Documents from the HawkIRB application.
IRB Advisor Newsletter, February 2021
By Kelly O'Berry, BS, CIP
IRB Advisor (a publication of Relias) is a monthly newsletter with articles about issues facing IRBs, Human Research Protection Programs (HRPPs) and researchers. Current and past issues of IRB Advisor are posted in the “IRB ICON Course for Researchers” which is accessible to anyone with an active UI HawkID. The portal to this ICON Course is on the Education and Training page of the Human Subjects Office website.
This month we highlight an article from the February 2021 issue about steps the research community can take to build trust among minority communities.
Research World Can Help Build Trust Among Minorities: Context Helps in Understanding
A history of unethical research among minority communities has led to a distrust of research and health care. This article suggests that researchers and IRBs need to listen to Black Americans and other minority communities to ensure equity and diversity in clinical trial participation. Pharmaceutical company sponsors may also need to provide access to healthcare for uninsured research participants beyond the time they are actively participating in the study. Building relationships can have a lasting impact on future research participation. It could also increase trust in the healthcare system and improve willingness to follow recommendations, such as receiving the coronavirus vaccine.
Additional articles in this issue:
- Research Trust Issues Affect Vaccine Rollout
- Ethical Considerations for Continued COVID-19 Vaccine Research
- Federalwide Assurance Revised: Responsibilities Under the Revised Common Rule
In the News
- Feasibility of Integrating Canine Olfaction with Chemical and Microbial Profiling of Urine to Detect Lethal Prostate Cancer, PLOS ONE
- ‘I Wanted to Go in There and Help’: Nursing Schools See Enrollment Bump Amid Pandemic, Kaiser Health News
- A Computational Reward Learning Account of Social Media Engagement, Nature Communications
- Meta-analysis of Neural Systems Underlying Placebo Analgesia from Individual Participant fMRI Data, Nature Communications


