HawkIRB Pushing Information to I-CTMS (Iowa Clinical Trials Management System)
On the evening of May 1, 2023 HawkIRB will automatically send information from submitted eResearch applications to the I-CTMS (Iowa Clinical Trials Management System). These programming integrations will save the research community from duplicating a significant amount of information found in HawkIRB into the I-CTMS. This automation will occur based on responses to new questions in HawkIRB.
HawkIRB has been integrated with the University of Iowa Health Care (UIHC), Holden Comprehensive Cancer Center (HCCC) clinical research services and their trial data management system, OnCore, for a number of years. Behind the scenes, HawkIRB has submitted a large amount of data from research applications to assist in the creation of a clinical trial record. On the evening of May 1, 2023, the Human Subjects Office, in partnership with the I-CTMS Administration Team for General Research, will be rolling out new changes to submissions for all non-oncology IRB-01 Biomedical and all External IRB submissions.
What is the I-CTMS?
The I-CTMS is a commercially available tool which has been used by HCCC for several years and was recently purchased by the UIHC for electronic clinical trial data management across the enterprise. The CTMS consists of multiple platforms to manage protocols, participants, financials, and regulatory documentation for clinical trials. The primary platform is called OnCore, however the University of Iowa has reimaged the CTMS for expansion to non-oncology use, and it is called I-CTMS. The I-CTMS is managed by the ICTS (Institute for Clinical and Translational Science) and is available to any University of Iowa researcher that would like to utilize an electronic clinical trial data management system. For more information and training opportunities on this tool, please visit the I-CTMS webpage on the ICTS website or contact ictms-admin@uiowa.edu.
HawkIRB and I-CTMS Integration
Phase I will involve two new questions for all IRB-01 and all external IRB applications. It will become increasingly important to ensure HawkIRB remains up to date on both IRB-01 and all external IRB submissions as information in HawkIRB is sent over to the I-CTMS. V.27 will ask whether or not the I-CTMS will be used for clinical trial data management. The use of the I-CTMS for clinical trial data management is recommended, but not required, for non-oncology clinical trials. The new question V.27 will only appear if V.22 asking whether or not Holden Comprehensive Cancer Center (HCCC) resources will be used is answered “no.” If V.22 is yes, oncology clinical trials will continue to use Oncore and the HCCC regulatory services for clinical trial data management. For all non-oncology clinical trials requesting to use the I-CTMS to fulfill their clinical trial data management needs, V.27 should be answered as “Yes.”

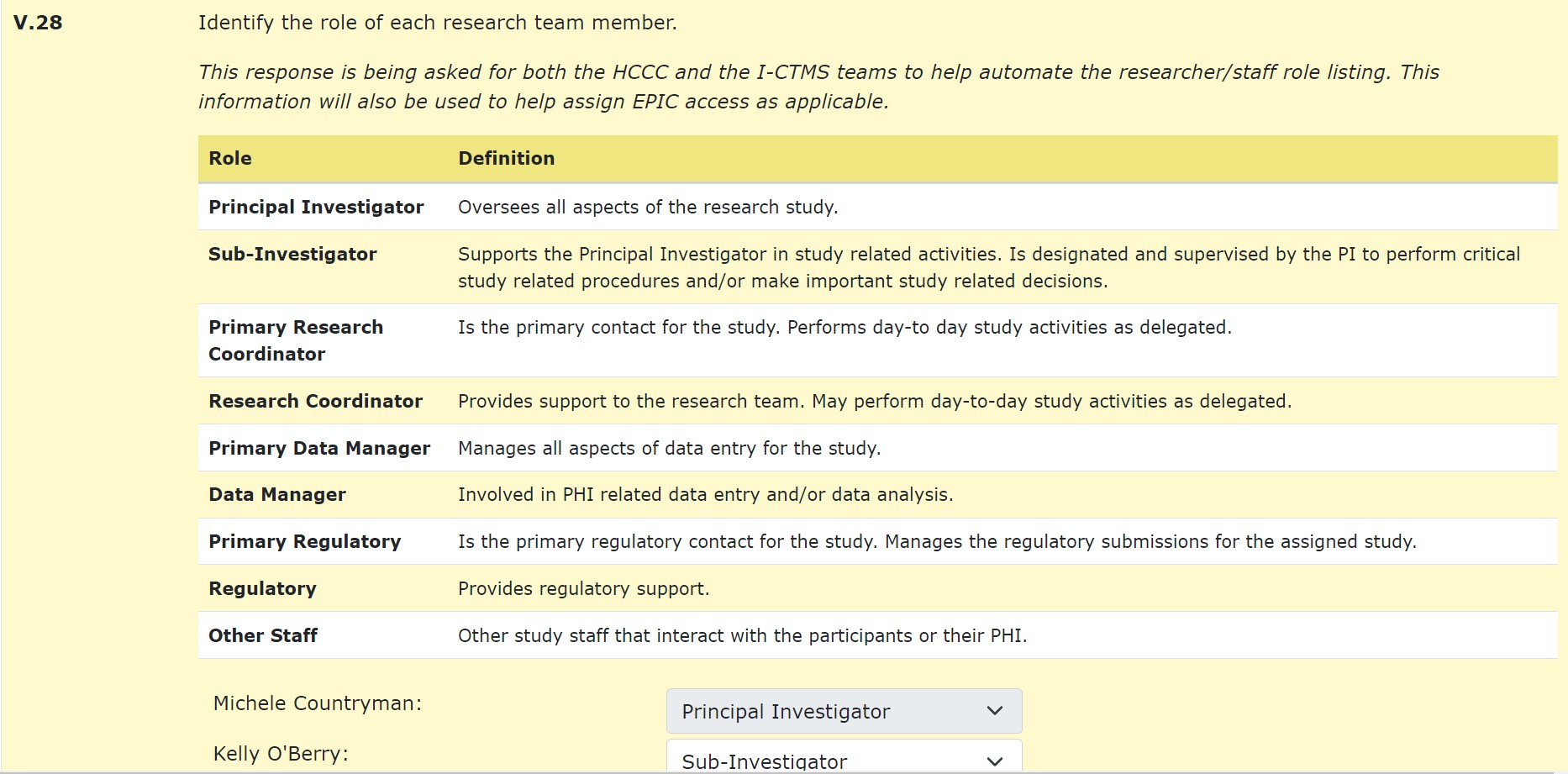
A subsequent question (V.28) will appear to assign staff roles to all listed research team members found in section II. These roles will be specific to responsibilities as defined in the I-CTMS and are not subject to IRB review or consideration. More information on the roles and responsibilities can be found in the training modules found on the I-CTMS webpage (under the Advarra University section)
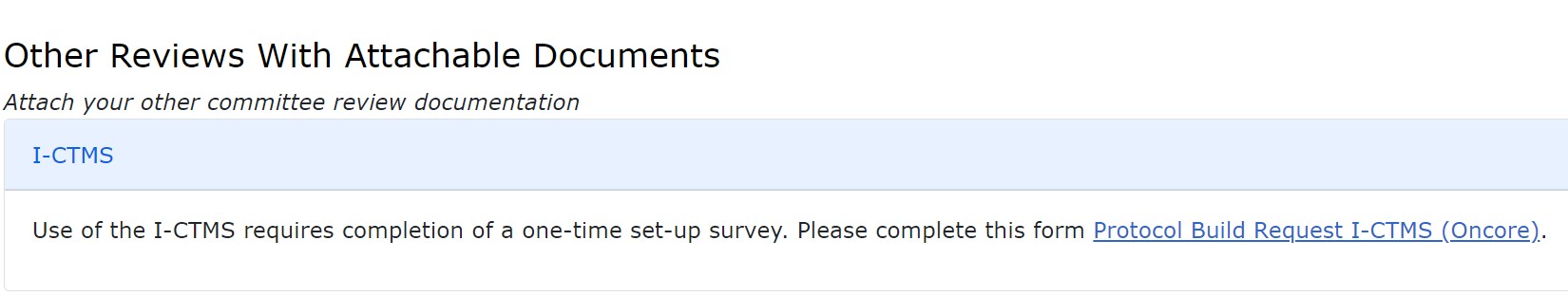
HawkIRB will also have an additional section with a link to complete an I-CTMS request form to initiate the clinical trial in the I-CTMS system. This REDCap form contains a short number of questions for information not found in HawkIRB. Completion of this form is required to initiate the I-CTMS study presence.
Once the HawkIRB applications is submitted to the HSO/IRB for review, HawkIRB will automatically push over data points into the I-CTMS to create the clinical trial shell. Once the IRB has approved the research and the project has been released to the study team, an additional push of information will be automatically sent to the I-CTMS to finalize the study clinical trial shell.
Study related information will continue to be pushed from HawkIRB to I-CTMS during the lifetime of the project until it is formally closed in HawkIRB. Here is a summary of information that will be pushed from HawkIRB to the I-CTMS during the lifetime of the study.
Data Pushed from HawkIRB to I-CTMS upon Submission
The following categories in the I-CTMS shell will be created based on the corresponding HawkIRB question responses.
|
I-CTMS Field |
HawkIRB Application Responses in Question # |
|
Protocol No. |
IRB ID# |
|
Department |
Based on PI role |
|
Organizational Unit |
V.27 |
|
Protocol Type |
VII.B.1 |
|
Title |
I.2 Project Title |
|
Age (Adults) |
VI.1 Adult Number |
|
Age (Minors) |
VI.6 |
|
Staff |
Section II and V.28 (assigned role) |
|
Form |
Form Type (New) |
This basic level information will initiate the creation of the I-CTMS shell. Once the new project application is approved by the IRB and all HRPP committee approvals are in place, the project is released to the researcher.
Data Pushed from HawkIRB to I-CTMS upon IRB/HRPP approval
HawkIRB will push an additional set of information from the HawkIRB application upon release from HawkIRB to the investigator to begin the research. Those fields include:
|
I-CTMS Field |
HawkIRB Application Responses in Question #/Process Point |
|
Submit Date |
Date the Form was Submitted to the HSO/IRB |
|
Committee |
IRB Type I.1 |
|
Summary |
XIII.1 (for Modifications forms) |
|
Institution |
University of Iowa-University of Iowa Hospitals and Clinics |
|
Protocol Fields
|
This includes:
|
|
Age |
Adult subject #s VI.1), Children subject #s (VI.6) |
|
Investigational Drug (Yes/No) |
Drugs listed in V.8 |
|
Research Team Member(s) |
II.1, II.2 and V.28 – this will be for both initial and all modifications when team members are added. |
|
Sponsor(s) |
III.1 |
|
IND ID |
IND Number (V.8) |
|
IND Holder Name |
IND Sponsor (V.8) |
|
IND Comments |
Generic Name (Commercial Name) (V.8) |
|
IDE ID |
IDE # (VII.B.27) |
|
IDE Holder Name |
Sponsor who holds the IDE (VII.B.28) |
|
IDE Comments |
IDE Name (VII.B.26) |
HawkIRB Attachments that will Push to I-CTMS
HawkIRB is set up to not only push data but will also include sending over several attached documents from the attachment page of the HawkIRB application into the I-CTMS on behalf of the study team. Those documents include:
|
I-CTMS Field |
HawkIRB Application Attachment Category |
|
Treatment Consent |
Document(s) attached in the “Informed Consent” attachment category |
|
Other |
Document(s) attached in the additional consent categories in HawkIRB |
|
Assent |
Document(s) attached in the Assent category |
|
Protocol or Study Design / Amendment |
Document(s) attached in the Protocol category |
|
Documents |
Document(s) in the Investigator Brochure category |
It is very important to make sure the naming scheme you use for attachment categories is concise and clear as the name of the attached document will now carry over from HawkIRB to the I-CTMS. See the February 2023 IRB Connection Newsletter to learn more about recommended naming schemes.
Ongoing data that will be pushed from HawkIRB to I-CTMS
Once the I-CTMS connection is developed, HawkIRB will push over project level data on both a form and study level in addition to the specific sections already noted. This information includes:
|
I-CTMS Field |
HawkIRB Application Responses in Question #/Process Point |
|
Review Date |
Last IRB Meeting Date (Full Board) or Date Approved (Expedited) |
|
Final form status |
Approved, Disapproved, Withdrawn, or Reviewed (Project Closure forms only) |
|
Review Reason |
Form Type (Mod, Mod/CR, CR, Closure Form) |
|
Review Type |
Full Board or Expedited |
|
Action |
Approved, Withdrawn, Disapproved, Closed |
|
Action Date |
Date Approved/Withdrawn |
|
Expiration Date |
Next Approval Due By Date |
What if I am already set up to use the I-CTMS?
Around 150-165 studies have already been working with the I-CTMS Administration Team for General Research team to create a clinical trial data management presence in the I-CTMS. Those studies have been preidentified in HawkIRB. The next time a modification is submitted to the HSO/IRB for review V.27 will appear as a new question. This question will automatically be answered “Yes” for any studies that were part of the initial back build pilot. V.28 will require a response as part of the modification. HawkIRB will continue to push information from HawkIRB to the I-CTMS as soon as the modification is approved.
Phase II Integration with HawkIRB and I-CTMS
Phase II integration efforts will focus on HawkIRB improvements when sending information to I-CTMS when:
- there is an external IRB of record.
- IRB-01 serves in the lead IRB role of a single IRB of record research initiative.
Programming enhancements have begun to better establish documentation of external IRB approved materials. Stay tuned for Phase II updates and announcements via the IRB Connection newsletter.
Questions?
If you have any questions regarding the I-CTMS or this transition, please contact the I-CTMS Administration Team for General Research at ictms-admin@uiowa.edu. If you have questions on HawkIRB or the HSO/IRB review process, contact the HSO at irb@uiowa.edu or (319) 335-6564.


