Index
Updates to Federal Regulations and Guidance
Requesting Feedback About IRB Educational Resources
HawkIRB Trainings, IRB Presentations, and Office Hours
IRB Education for Your Group: Presentations to Suit Your Needs
Herky Hint: HawkIRB Delegate Permission System
IRB Advisor, January 2018- Revisiting the “Unfortunate Experiment” in New Zealand
From the Director
eResearch (HawkIRB) Inbox Enhancements
Effective February 4th: As a result of feedback from the Human Research Protection Program (HRPP) survey administered by the Vice President for Research and Economic Development and the Human Subjects Office in April 2017, several enhancements have been added to the eResearch (HawkIRB) inbox view. These changes are specific to eResearch projects currently undergoing, or pending, HRPP and IRB review. eResearch projects in this category are defined as projects submitted for review to the HRPP\IRB but not yet approved. We hope the enhancements will provide a quick “at a glance” update on the status of the eResearch review. It is also our hope these updates provide greater transparency to the overall HRPP and IRB review processes.
First an orientation to what you see in your HawkIRB inbox: There are four primary sections of the HawkIRB Inbox. These sections only show when you have projects that fit in these categories:
- Inbox – This section includes any projects returned by the HSO\IRB which require a response in order to continue moving forward with review of the IRB application. Research projects listed here are not considered to be in the HRPP\IRB Review que until all requests have been addressed by the Principal Investigator or their delegate. Once the request(s) is\are addressed, the project requires routing back to the HSO\IRB for further processing. No enhancements were made to this section.
- Draft Forms – This section which include any projects initiated but not submitted to the IRB. No enhancements were made to this section.
- Pending Forms - This section captures all research projects currently undergoing review by the HRPP\HSO\IRB. The current view lists limited information such as the IRB ID#, IRB Type, Study Title, Form Type, and the ability to “review” the research project submission in its entirety. This is the section that has been updated to show the current basket for the IRB review process and the status of any Other HRPP Committee reviews.
- Projects – This section contains a full list of research projects released to the PI with full HRPP\IRB approvals in place. No enhancements were made to this section.
Below is an illustration of the updates.
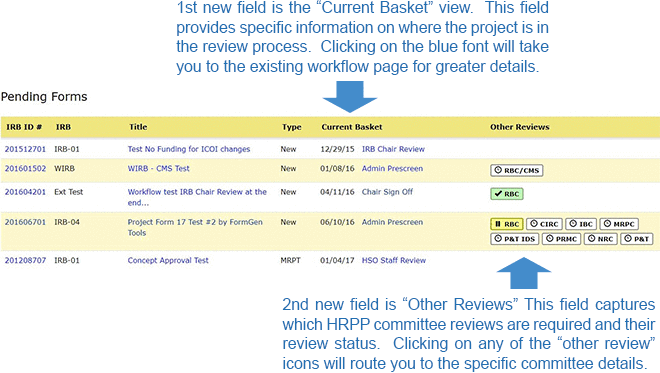
Information on the HSO\IRB Review process as identified in the first new field above and a definition of workflow routing “Baskets” can be found on the FAQs found on the HSO website under “Status of a Submitted Application.” The HRPP committees appearing in the second new field above are required reviews based on how Section V of the eResearch application is completed. The color scheme used to represent status is the same as what you are accustomed to when completing the eResearch application.
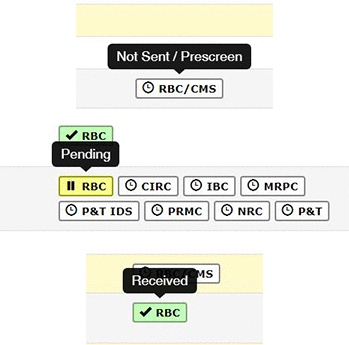
- White (with a clock icon) means the committee review is required but the project is in our Administrative prescreen so the HRPP committee has not been notified of the eResearch project.
- Yellow (with the Pause icon) means the HRPP committee has been notified and the review is underway but not complete.
- Green (which the checkmark icon) means the HRPP committee has finalized their review and approval has been issued.
There is also an ability to hover over each HRPP icon to display a verbal status update.
More Information on the HRPP review process can be found on the HSO Website under the “Other Research Review” menu link and on the Human Research Protection Program Flowchart. We hope you will find these enhancements helpful. If you have questions or have other feedback you would like to provide regarding the HRPP\IRB review process, please contact me in the Human Subjects Office via email irb@uiowa.edu or (319) 335-6564.
Best Regards,
Michele Countryman
Updates to Federal Regulations and Guidance
By Kelly O’Berry
Federal regulatory agencies announced several updates in the last few months. These updates from the Office of Human Research Protections, the Food and Drug Administration and the National Institutes of Health are summarized below and posted on the Guidance and Regulation Updates page of the Human Subjects Office website.
Office of Human Research Protections (OHRP)
- Changes to the Regulations for the Protection of Human Subjects – Perhaps the biggest news is the January 17, 2018 announcement of a six-month delay in the effective and compliance dates for the changes to the Common Rule (45 CFR 46) that were first announced on January 18, 2018. These regulations were set to take effect on January 19, 2018 but have been delayed until July 19, 2018. The UI Institutional Review Board is preparing to revise our policies, procedures and HawkIRB forms in accordance with these regulatory changes.
Food and Drug Administration (FDA)
- Research Subject Compensation – On January 25, 2018, the FDA revised the current guidance about paying research subjects. The updated guidance is now called “Payment and Reimbursement to Research Subjects” and specifies that the IRB should consider reimbursement for travel, airfare, parking and lodging separately from payment for participation that must be reasonable in relation to their time, inconvenience, discomfort or other considerations about their participation in the research. The FDA does not consider reimbursements for travel and lodging to present an undue influence for subjects to participate in the research. But the research subject compensation for participation must be “just and fair” and should be neither coercive or present an undue influence.

National Institutes of Health (NIH)
As a reminder, the following NIH policies went into effect on January 25, 2018. For more information, follow the links below to the notices on the NIH website.
- New Review Criteria for Research Project Applications Involving Clinical Trials – In September, 2017, NIH announced plans for additional review criteria for all clinical trial applications submitted on or after January 25, 2018. NIH has expanded the criteria for what constitutes a clinical trial. They now include any study in which human subjects are prospectively assigned to one or more interventions to evaluate their effect on health-related biomedical or behavioral outcomes. These interventions could include a placebo or other control. Follow these links to the NIH policy and to a tool posted on the NIH website to determine if an NIH-sponsored research study meets the new definition of a clinical trial.

- Use of a Single IRB for Multi-Site Research – In June 2016, NIH announced the expectation for the use of a single IRB (sIRB) for NIH-funded protocols conducted at more than one site in the United States. This policy applies to all applications for NIH funding that are submitted on or after January 25, 2018. Follow these links to the final policy and to a post on the NIH website that outlines the purpose, guidelines and resources regarding this new policy.
The Human Subjects Office will continue to provide updates via the IRB Connection Newsletter about regulatory and policy updates that affect the UI research community.
Requesting Feedback About IRB Educational Resources
By Kelly O’Berry


The IRB Connection Newsletter is just one of many educational resources offered by the IRB Education & Outreach Group in the Human Subjects Office. We hope the content has been useful for you. We would very much appreciate your feedback about all of our educational resources to help us provide guidance and support that meet the needs of the UI research community in the coming year. Click here to complete a very brief (2 minute) feedback survey if you didn’t already complete the survey when we originally sent it out in December 2017.
You may also contact me directly at kelly-oberry@uiowa.edu or 319-335-8477.
HawkIRB Trainings, IRB Presentations, and Office Hours
By Brent Collinsworth
Welcome to the Spring 2018 semester! We have posted the schedule of HawkIRB trainings, IRB Presentations, and office hours on our website. You can find more information on each of these by clicking on the links above.
HawkIRB Trainings


We offer a 3-part series of HawkIRB trainings once a month. These trainings provide information on how to navigate the HawkIRB platform and guidance on how to complete questions on the New Project Application. Part 1 of the training covers basic navigation information and Sections I-VI of the New Project Application. Part 2 covers Sections VII-XII and the Attachments page. The After IRB Approval training is about the forms submitted after receiving IRB approval for a New Project Application, including Modifications, Continuing Reviews, Reportable Event Forms, and Project Closure Forms. For more information on these trainings or to pre-register, follow the link here.
IRB Presentations
Each semester, the Human Subjects Office offers a variety of presentations on topical issues related to research and the IRB. These presentations are open to the entire research community. This semester, we will offer presentations on Data Usage Agreements, Expanding the Concept of Vulnerability, Best Practices in Research Compliance, Best Practices in Privacy and Confidentiality Protections and Record Storage, and a moderated panel on Gender Identity and Research.
Office Hours
Finally, the Human Subjects Office offers open office hours to the research community. Office hours are designed to be a time where researchers can ask questions about the ethical conduct of human subjects research and about HawkIRB application. For more information on Office Hours, follow the link here.
Registration
We recommend pre-registration for the HawkIRB trainings and IRB presentations to ensure space and available handouts. There is a link to register on each page. But IRB Office Hours are first come first served; no appointment necessary. We hope to see you soon at one of our HawkIRB trainings, IRB presentations or at IRB Office Hours.
Resources for New Researchers
By Kelly O’Berry, BS, CIP


The IRB Education & Outreach Group offer support and guidance for new human subjects researchers. These resources may be useful whether you conducted research at another institution or if you’re just planning to do your first study here at the University of Iowa. New Researchers or departmental support staff may contact us at irb-outreach@uiowa.edu to request an IRB Orientation. A member of the Education and Outreach Group will meet with new researchers to provide an overview of the IRB review process and orientation to the electronic IRB application system, HawkIRB. We can guide you toward obtaining any additional approvals from other committees in the UI Human Research Protection Program that may be necessary for your research.
We also strongly encourage new researchers, research team members and HawkIRB delegates to take advantages of the following educational resources:
- HawkIRB Trainings – We offer a three-part training series to teach researchers and their delegates how to navigate the HawkIRB system and provide thorough, detailed responses to questions in the HawkIRB forms.
- IRB ICON Course for Researchers – If you are not able to attend the live HawkIRB training sessions, or if you need a refresher on a particular session, there are separate recordings about each section of the HawkIRB application posted in our IRB ICON Course. This course is available to anyone who has a HawkID and password.
- IRB Connection Newsletter – The Human Subjects Office publishes a monthly newsletter for the UI research community. Each issue includes several articles, announcements about upcoming educational opportunities and highlights of research-related articles in the news. We send the newsletter to everyone who is a Principal Investigator or research team member for a study in HawkIRB and to all HawkIRB delegates.
- Human Subjects Office Staff – The Human Subjects Office (HSO) staff may be one of the best resources for new researchers. You can contact us with questions or to request assistance in any of the following ways:
- Attend IRB Office Hours – During the Spring and Fall semester we hold IRB Office Hours three days a week. Mondays in S108 Lindquist Center (2-4 pm), Tuesdays in 101 Hardin Library for the Health Sciences (2-4 pm) and Wednesdays in 101 Hardin Library (10 am-12 pm). In the summer (June-August), IRB Office Hours are on Wednesdays (2-4 pm) in 101 Hardin Library.
- Call the Human Subjects Office – You may contact us by phone at 319-335-6564. Phones are answered Monday through Friday from 8 am-12 pm and 12:30-4:30 pm. If we don’t answer, please leave us a message so we can call you back.
- E-mail the Human Subjects Office – You may contact us by email at irb@uiowa.edu.
IRB Education for Your Group: Presentations to Suit Your Needs
By Brent Collinsworth


The IRB Education & Outreach Group offers many educational resources to the UI research community. As a reminder for the Spring 2018 semester, we want to highlight a resource that may be especially useful to instructors of research methods courses, other research-related courses, journal clubs and orientation sessions or seminars for new faculty, graduate and professional students. The IRB Education and Outreach program is available upon request to give classroom and group presentations about the ethical conduct of research and the UI IRB review process. We can tailor the content to suit your needs, and we are able to present to a wide variety of courses and groups, including evening and online classes.
We offer a general IRB overview presentation that provides a broad overview of topics that are relevant for students and others who plan to conduct research themselves, whether as a course requirement or as an honors, Master’s or doctoral dissertation. This presentation can cover some or all of the following:
- The definition of human subjects research
- How to ask if you need IRB approval
- Ethical conduct of research (the Belmont Report)
- The UI IRB review process
- Informed consent
- Research off campus or outside the United States
- Course-related student projects
- IRB Resources that are available for UI/VA researchers
This presentation provides a good orientation to the UI IRB and the IRB approval requirements for faculty, staff and student researchers.
We also offer a full presentation about the ethical conduct of research. It includes an in-depth discussion of the ethical principles of human subjects research that are outlined in the Belmont Report and examples of unethical research conducted both historically and recently. This presentation is designed to help students apply basic ethical standards to real-world examples of human subjects research. It would be an excellent supplemental lecture for any research methods course.
Each of these presentations typically fills a 50-minute class period, but we can tailor the length and topic to suit the needs of the class or group. Contact us at irb-outreach@uiowa.edu to request a presentation.
Herky Hint: HawkIRB Delegate Permission System
By Kelly O’Berry, BS, CIP


The HawkIRB System has a feature that allows Principal Investigators (PIs) to name delegates to act in the system on their behalf. A delegate has the same permissions in HawkIRB as the PI; to create and submit new forms, respond to workflow, attach and edit documents. Delegates receive automated messages from the HawkIRB system; notices about submitting Continuing Review forms, and that the IRB routed a form back to the PI in workflow or approved a form.
The Delegate Permission System lets the PI assign access for delegates to certain studies and restrict access to other studies. This feature is the most useful when a PI has multiple open studies. When the permission system is turned on, a PI can specify the delegates that receive notices and have access to each study.
We really want PIs and their delegates get the most out of the Delegate Permission System by knowing how to add or remove delegates, turn on the Delegate Permission System and act as a delegate for a PI.
Add or Remove Delegates
Only a PI can add or remove a delegate. A delegate cannot add themselves or name other delegates for the PI. Follow these steps to add a delegate:
- Click on the “Personalize” tab on the black tool bar
- Select “Update my delegates”
- Enter the HawkID of the person you wish to add as a delegate. If you don’t know their HawkID, click on the “Campus Directory” link and look them up in the UI directory. Copy their HawkID and paste it into the open field.
- Click the gray “Add Delegate” button
To remove a delegate, click on the “remove” link for the delegate on the right side of the screen. Be aware that the system does not ask if you really mean to remove them. They are immediately removed. But if you remove someone by accident, just follow the directions above to add them again.
Turn on the Delegate Permission System
Follow these steps to turn on the system:
- Click on the “Personalize” tab in the black tool bar
- Select “Update my profile”
- Select “Yes” under “Turn on the Delegate Permission System”
- Go back to the “Personalize” tab
- Select “Update my delegates”
- For each delegate, make a selection for what happens “When a delegate opens your inbox:” they either see all projects (even the ones they cannot edit) or you can hide the projects that they don’t have permission to edit.
- Click the box next to each study to grant them access.
- Make sure you scroll all the way down and click the gray box to “Save Permission Information” (otherwise your changes will not be saved when you navigate away from this screen)
If there are changes in the role of a delegate or the studies in which they are involved, simply follow steps 5-7 above to make changes in your selections. Additional information is available on this Frequently Asked Question (FAQ) page about the HawkIRB Delegate Permissions.
Acting as a Delegate for a PI
Delegates should always log in to HawkIRB with their own HawkID and password. For security reasons, do not use the PI’s HawkID and password! After you log in, click “delegate login” in the upper right corner of the screen, to the right of your name. You will see a “login” option on the right side of the screen for each PI that has named you as a delegate. Follow that link to login to the PIs inbox.
When a New Project form is created in someone’s HawkIRB inbox, that person is the PI. At least once a month we receive calls or emails from delegates who accidentally created a New Project form without logging in as a delegate for the PI. When that happens, the delegate is the PI on that form. Unfortunately, there is no easy way to transfer a draft form from one HawkIRB inbox to another. When this happens, the only solution is to start a new ‘New Project’ form in the correct persons’ inbox. You can use the Printer Friendly View of the first form to copy and paste content into the new form. Additional information is available in Question 3 on this FAQ page.
The PI is ultimately responsible for the content of the HawkIRB application. It is essential for the PI to review each form and all attached documents before submission to the IRB. The PI should also be involved in the workflow communications during the IRB review process.
Additional information is available in this FAQ about Delegates in HawkIRB.
IRB Advisor, January 2018- Revisiting the “Unfortunate Experiment” in New Zealand
by Brent Collinsworth


Most students who study research methods and ethics only learn about incidents of unethical research that occurred in the US as a part of their curriculum. However, egregiously unethical experiments have occurred around the world, and these studies often receive little attention in the US. One of these is the so-called “Unfortunate Experiment” at the National Women’s Hospital in Auckland, New Zealand. It began in 1966 and continued until a 1987 expose in Metro Magazine. Dr. Herbert Green, a gynecologist at National Women’s, did not believe that cervical carcinoma in situ, a grouping of abnormal cells in otherwise normal tissue, led to the development of cervical cancer.
Dr. Green did not provide treatment to a group of women diagnosed with CIS and followed their progress to see if they developed cancer. Green did not inform the women about their diagnosis, and enrolled them in his study without their knowledge or consent. In a chilling parallel to the Tuskegee Syphilis Experiment, the hospital let the research continue even after a 1984 study published by one of Green’s colleagues, showed a clear link between untreated CIS and cervical cancer. The unethical nature of this study also led to the establishment of a Code of Health Consumers’ Rights and the formation of the first ethics committees in the country.
In this article, “Revisiting the ‘Unfortunate Experiment’ in New Zealand,” the IRB Advisor interviewed Dr. Ronald W. Jones, a doctor at National Women’s Hospital who published the 1984 paper on the link between CIS and cancer. They discuss his experiences after publishing the experiment, the culture of denial surrounding the harm the Unfortunate Experiment was causing, and his new book, “Doctors in Denial: The Forgotten Women in the Unfortunate Experiment.”
Other articles in the January issue of IRB Advisor include:
- “Clinical Trials Often Exclude Women, Even When There Could Be Compelling Benefit”
- “How to Ensure Exempt Studies Maintain Data Confidentiality”
- “Study Looks at Use of Emergency Research Informed Consent”
- “Counterpoint: Transplant Recipients of Research Organs Entitled to Informed Consent”
- “OHRP and FDA Issue Guidance on IRB Meeting Minutes”
- “Purdue IRB Shuts Down Youth Study”
Current and Past Issues
There is a link to current and past issues of IRB Advisor on the Education and Training page of the Human Subjects Office web site. This link provides automatic access to the newsletter from all computers with a University of Iowa IP address.
The University of Iowa username and password cannot be posted on the Human Subjects Office web site. UI researchers may contact the Human Subjects Office to request the username and password to access IRB Advisor from a personal computer. Contact us by e-mail (irb-outreach@uiowa.edu) or call us at 319-335-6564.
Continuing Education Credits
Individual newsletter subscribers can receive 1.5 AMA PRA Category 1 Credits™ or 1.5 nursing contact hours for reading an issue and completing an online test. However, since this is an institution-level subscription, UI researchers must purchase access to an individual issue for $40, or purchase a full subscription, to receive CME/CE credits. Visit the AHC Media web site for information about subscription options.


