Index
Corporate Funded Clinical Trial F&A Rate Increase (***Rebroadcast***)
Holiday wishes from the HSO
Is there a recording of that IRB presentation I missed?
Herky Hints: HawkIRB Workflow
Medical Ethics Advisor Newsletter, October and November 2021
In the News
Corporate Funded Clinical Trial F&A Rate Increase (***Rebroadcast from Office of the Vice President for Research (OVPR) and the Division of Sponsored Programs (DSP) ***)
The Human Subjects Office (HSO) is rebroadcasting the corporate funded clinical trial F&A increase notice sent on 12/9/21 from DSP as it contains important information impacting human subjects research. Information regarding the F&A increase, effective January 1, 2022, can be found on the DSP website. This increase will be used to support a new enterprise clinical trial management system (CTMS) that will be compliant with the FDA 21 CFR 11 regulations.
The HSO has long term plans to collaborate with the UI to further integrate HawkIRB (eResearch application) with the CTMS. Integration plans will continue to be announced via the IRB Connection.
If you have questions regarding
- F&A costs, see: F&A Costs on DSP Website
- Corporate and Industry Sponsored policy, see Grant Accounting Office website
Holiday wishes from the HSO
Warm wishes to you and your family during the holiday season. We look forward to working with you in 2022!
Is there a recording of that IRB presentation I missed?
By Kelly O’Berry, BS, CIP
In order to support new staff onboarding and continuing education within the UI and VA Health Care System research community, recordings of IRB presentations are posted in the Additional Topics section of the IRB ICON Course for Researchers.
Orientation to the IRB ICON Course for Researchers
The portal to the IRB ICON Course for Researchers is on the Education and Training page of the Human Subjects Office (HSO) website. ICON is the Learning Management System the UI uses for academic courses. The IRB ICON course is available to anyone with a HawkID so all members of the UI and VAHCS research community should have access to this course.
The first module is a table of contents. Click on each item to read a brief overview of what you will find in the subsequent modules.
- Student PI Training Requirement
This module includes recordings of the 4-part HawkIRB training series. Student Principal Investigators (PIs) must view the recordings of the HawkIRB New Project trainings (Part 1 and 2) and pass the quizzes before they can submit a HawkIRB New Project form to the IRB. However, this module is NOT just for students! Anyone can view these recordings, but you will need to view the full Part 1 recording and pass the quiz to access the Part 2 recording. There are no pre-requisites for the Part 3 and Part 4 recordings.
- HawkIRB Training Quick Tutorials
Although these trainings are over 11 years old and we have updated the HawkIRB forms in that time, the format of these recordings may be useful for researchers who just want to learn about the HawkIRB system, a specific section of the New Project form or other forms you submit after IRB approval.
- Additional Topics
This is a warehouse for recordings of past IRB presentations. They are posted in reverse chronological order. Whenever possible, we post the presentation synopsis and pdf of the slides. Researchers can use CTRL-F to search the page for key words, to see if there are past presentations on certain topics.
- Medical Ethics Advisor Newsletter (formerly IRB Advisor)
The Human Subjects Office subscribes to a national newsletter that has articles about medical ethics. The IRB Advisor newsletter addressed IRB-related topics, but it was discontinued in August 2021. The new Medical Ethics Advisor newsletter covers a broader subject matter, but every issue has several articles about IRB-related topics.
Recorded Presentations
The following recordings were recently posted in the Additional Topics tab:
- Exempt Application Updates – from November 2021
- Hot Topics and Updates – from July and October 2021
- Research Billing Compliance – from September 2021
There are other recorded presentations about the external IRB review process, student PI and Faculty Advisor (FA) responsibilities, managing HawkIRB workflow, data security, and use of eConsent, just to name a few.
We want to call special attention to the 3-part lecture series “Getting on the Same Page: Submission and Review of Human Subjects Research Applications.” This series was presented in collaboration with the Division of Sponsored Programs (DSP) and the Institute for Clinical and Translational Science (ICTS). This lecture series addressed how to achieve the most efficient review of human subjects research by the Human Research Protection Program (HRPP).
Herky Hints: HawkIRB Workflow
Joanie Hoefer, BS CIP
Workflow is a system in HawkIRB that allows Human Subjects Office (HSO) staff, IRB members, and researchers to communicate back and forth about a form that is in the review process. Workflow is used by the IRB to send any questions and/or requests for clarification to the investigator, and the investigator uses workflow to respond to these questions and clarifications. This article will provide a tour of the workflow page and best practices when responding to workflow questions.
When HSO review staff or an IRB Chair sends a form back to a Principal Investigator (PI) in Workflow, it will appear in the PI’s HawkIRB Inbox. The PI, his/her HawkIRB delegates and all contact persons listed on the research team receive an email notification from HawkIRB indicating that a form has been returned to them. HawkIRB will also send these individuals reminder emails every two weeks if the study is not returned to the IRB in a timely manner.
The Workflow Page
When you receive a form back in Workflow, it will appear in the Inbox section in bolded text. To look at the reviewers questions, click on the “wkflw” link to the far right of the application title. Once on the Workflow page, on the top left of the page, you will see the title of your study, the IRB ID number, the type of the form this workflow is for (New Project Application, Modification, etc.) and options to view or edit the form. Clicking on the “edit” link here will take you to the Application Index for the form. On the top right of the page, you will find important information about the Workflow system, how to navigate it, and what to do when you complete all actions and need to return the form to the HSO.
Below this information are numbered correspondence items requesting additions, changes or clarification to the HawkIRB form. Typically, there is a link to the specific section of the HawkIRB form to make the change. Workflow items follow a similar format, shown in the example below:
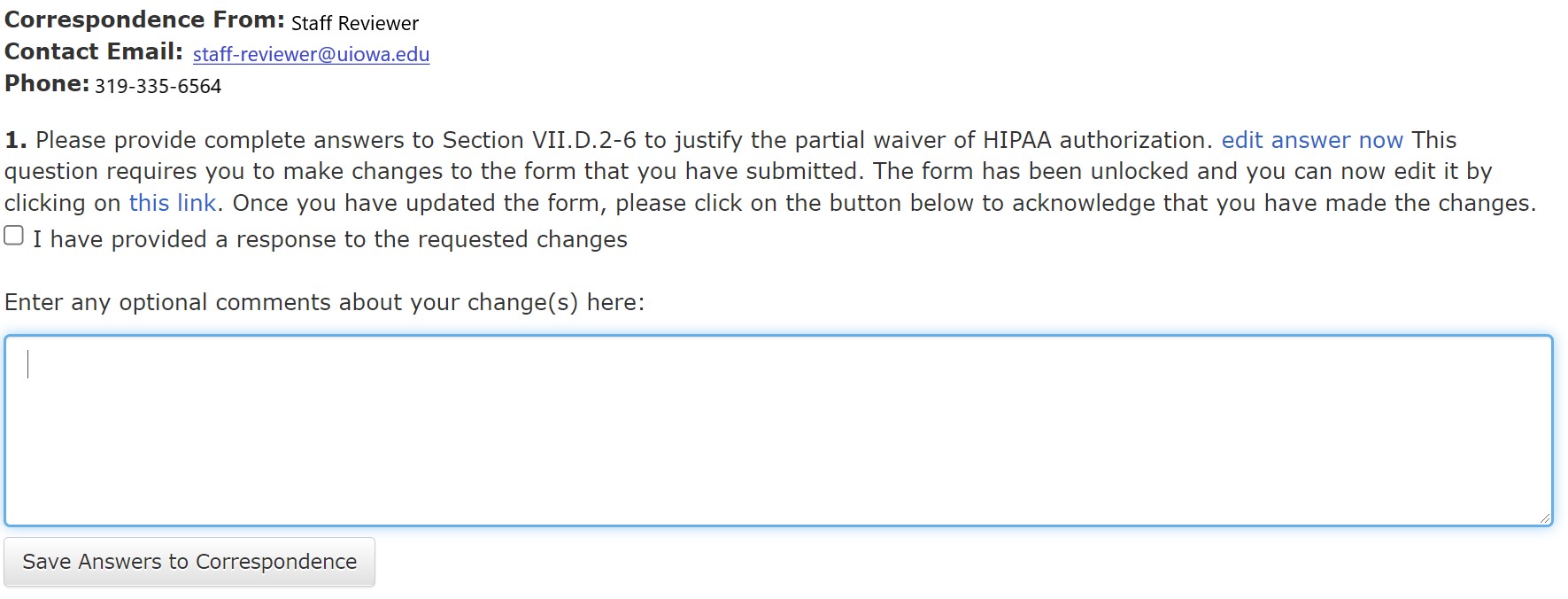
Each workflow item is sequentially numbered even if a form is sent back and forth between the PI and HSO/IRB several times. Following the workflow items and toward the bottom of the workflow page, there is a link to view previous workflow correspondence:
Finally, at the bottom of the screen is an open text box for optional comments, as well as the link to “Return Routing Slip to the HSO” after you have made the requested changes:
Best Practices for Using Workflow
Now that you understand how to look at the Workflow system, here are some helpful hints when working in Workflow:
- Make changes in response to questions in the HawkIRB form.
It is not enough to provide additional information in the comment section in Workflow. The full study description must be in the HawkIRB application form. The “Edit answer now” link will send you directly to the HawkIRB question referenced in Workflow.
- Address all items on the workflow form.
If you skip workflow items or do not fully address them, the staff reviewer will send them again in another round of workflow. Your application will go through the review process more quickly if you address all questions the first time.
- Ask for clarification or assistance.
The workflow page has the staff reviewer’s email and phone number. If you have any questions or comments about the item in workflow, don’t hesitate to contact that staff member.
- Remember to save all changes in the form.
Once you have made any necessary edits, click on the “Back/Save,” “Index/Save,” or “Continue/Save” button on the top or bottom of the application page in order to save the changes.
- Getting back to workflow questions.
Once you save the changes made to your application, you can click on the workflow shortcut link at the top right of your screen by your name to get back to the workflow item. You can either click on the file folder to go back to the workflow screen OR right click the file folder and select “Open in a new tab” if you want to alternate from the workflow requests and making edits in the HawkIRB application. This allows you the ability to work from two views in HawkIRB and is best utilized if you are working from dual monitors.
- Let the IRB know that you addressed each Workflow question.
Verify that each item was addressed and then click on the “I have made the requested form changes” box by each Workflow item. The HawkIRB system will not let you send the form back to the HSO/IRB without checking this box.
- Limit changes to only address items in workflow, if possible.
Additional changes beyond the ones requested by HSO staff or IRB members may delay the review. Of course, go ahead and make additional changes if there is an error in the description of study procedures or the procedures have changed since you submitted the application.
- Click the “Return Routing Slip to the HSO” button once you have addressed all Workflow questions.
Once your application has been sent back to you in workflow, review on your study stops until you route the application back to the HSO. Address the comments and return it as soon as possible to continue the review process.
Addressing requested changes in the Workflow system promptly and accurately is key to keep IRB review of your study moving forward. If you have any questions about specific workflow items, it is best to contact the HSO staff reviewer directly. If you have general questions about the HawkIRB forms, don’t hesitate to contact the Human Subjects Office at irb@uiowa.edu or 319-335-6564.
(This article was adapted from an original article that was written by Brent Collinsworth and appeared in the April 2018 edition of the IRB Connection newsletter.)
Medical Ethics Advisor Newsletter, October and November 2021
Joanie Hoefer, BS, CIP
Medical Ethics Advisor (formerly called IRB Advisor, a publication of Relias, LLC) is a monthly newsletter with articles about human subjects research and medical ethics. Current and past issues of Medical Ethics Advisor and IRB Advisor are posted in the “IRB ICON Course for Researchers” which is accessible to anyone with an active UI HawkID. The portal to this ICON Course is on the Education and Training page of the Human Subjects Office website.
This month we are spotlighting articles from the October and November 2021 Medical Ethics Advisor Newsletters.
Quality Improvement Project Reveals Reasons for Long IRB Approval Process (October 2021 Medical Ethics Advisor)
A recent analysis of protocols was conducted by researchers from Columbia University to find out what caused the most delays in receiving IRB approval. The researchers looked at studies that required at least 2 IRB full board reviews before being approved and other protocols that were reviewed faster. The main reason for delays in IRB approval was that the researchers had not provided protocols that adequately described the research to the IRB. They also discovered that communications from the IRB could be difficult for researchers to understand.
The research demonstrated that numerous little inefficiencies on the part of both the researcher and the IRB can add up and lead to significant delays in IRB approval.
Some Researchers Turn to Social Media Influencers for Help with Recruitment (November 2021 Medical Ethics Advisor)
A small number of researchers are using the popularity of social media “influencers” to help recruit participants for research studies. Influencers generally have thousands of social media followers that look to them for advance or word-of-mouth information. Using them has helped diabetes researchers reach specific populations that they normally would have a difficult time accessing.
This type of recruitment takes time, however. It takes time to develop rapport with the influencer so that they agree to work with the researcher. If the researcher is not particularly social media savvy, they will need to get comfortable with the platforms before they pursue this method of finding potential participants. It may also require additional, proactive conversations with the IRB who may have reservations about using someone on social media to spread the word about a research study.
Additional Articles in the October 2021 Issue:
- IRBs Facing Ethically Controversial Questions on Brain Research
- To Eliminate Race-Based Disparities, Start by Asking Questions
- IRBs Use Inconsistent Processes for Informed Consent with Non-English Speakers
- Ethical Concerns on Conscience Clauses in Genetic Counseling
- IRBs Scrutinizing Recruitment of Adolescents via Social Media
- Minority Residents’ Palliative Care Training Quality Trails Other Medical Education
- Needlessly Delayed IRB Approval Raises Ethical Concerns
- Data on Ethics Programs Fill Knowledge Gap
- Qualitative Methods Give Unique Insights on Ethics Consult Standards
Additional Articles in the November 2021 Issue:
- IRBs Now Expect More Diversity in Research Trials
- Report: Still Not Enough Women Included in Cardiovascular Research
- Bioethics Field Lacks Standardized Competencies for Trainees
- Patients, Family, Clinicians All Misunderstand Chaplains’ Role
- Study: More Than Half of DNR ED Patients Resuscitated Against Their Wishes
- Ethics Service Uses Relative Value Units to Quantify the Work of Consultants
- Ethics of 10-Year Research Agenda for Dementia, Alzheimer’s Studies
- The Earlier, the Better for Formal Ethics Training
- IRBs Face Unique Ethical Questions About Disaster Research
- Protocols for Scarce Resources Draw on Ethical Principles, Empirical Data
In the News, December 2021 Bonus Edition